DIVISION OF PLANT QUARANTINE

Head of Division: Dr. Celia Chalam Vasimalla
Contact Numbers: 011-25841457, Extn. 787 ; +91-9968299994
Address: ICAR-National Bureau of Plant Genetic Resources, Pusa Campus, New Delhi – 110012, INDIA
E-mail: celia.chalam-icar@nic.in, mailcelia@gmail.com,nbpgr.quarantine@gmail.com
Mandate
Plant quarantine is a legislative measure enforced to regulate the introduction of seeds, plants/ planting materials, plant products, soil, living organisms etc. in order to prevent inadvertent introduction of insect pests, nematodes, pathogens (fungi, bacteria and viruses) and weeds harmful to the agriculture/ state/ region, and if introduced, prevent their establishment and further spread. Transboundary movement of plant material carries the risk of entry of the associated pests. NBPGR has been given the responsibility on behalf of the Government of India to carry out quarantine checks on the plants/ planting material meant for research purposes for both public and private sectors. Detailed quarantine examination is carried out for fungi, bacteria and viruses, insects, nematodes and weeds.
MANDATE- Quarantine Processing of Plants/ Planting Material under Transboundary Exchange
- Pest-free Conservation of Indigenously collected Germplasm
- Supportive Research to Develop Techniques for Detection and Salvaging of Germplasm
- Policy Issues on Related Biosecurity Issues
- Human Resource Development
Salient Achievements
- A total of 6,43,885 samples of various imported crops were processed for quarantine clearance during April 2019 – March 2024 of which 27209 samples were found infested/ infected with various pests (insects/ mites- 13,998, nematodes- 2,147 fungi/ bacteria- 10102, viruses 836, weeds- 126).
- Among infected samples 25,872 samples were salvaged by various techniques and treatments and rest (1,337) were rejected. Samples of some crops were given prophylactic treatments viz., fumigation (1,66,927), hot water treatment to paddy (17,753), pesticidal dip/spray to vegetative propagules (13,183) which also includes formaldehyde root dip treatment to rooted material against nematode pests, and 10% tri-sodium orthophosphate treatment (4,073) to capsicum, tobacco and tomato.
- A total of 162 pests economically important pests were intercepted comprising insects (20), fungi (58), bacteria (1), viruses (31), nematodes (9) and weeds (43). Of these 49 pests comprising insects (5), fungi (4), viruses (14), and weeds (26) are not yet reported from India. Nine pests comprising fungi (1), viruses (7) and nematode (1) are not reported on the host in India.
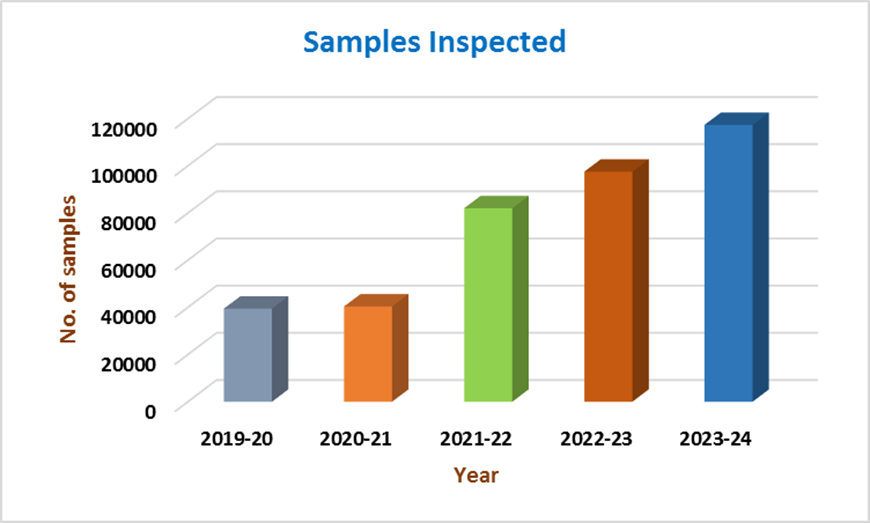
- A total of 197 post-entry quarantine inspections were carried out at various indenters’ sites that included 376,388 samples of various crops were inspected for presence of exotic pests at indenter’s sites (Fig 1 & 2).
- A total of 5,870 samples of exotic germplasm comprising Glycine max (1522), Phaseolus acuitifolius (2), coccineus (4), P. lunatus (54), P. vulgaris (1277), Pisum fulvum (1), P. sativum (266), Psophocarpus tetragonolobus (8), Vicia faba (367), V. sativa (10), Vigna spp. (76), V. radiata (700), V. trilobata (2), V. mungo (8), V. umbellata(102), V. unguiculata (1,436), V. unguiculata subsp. sesquipedalis (35) were grown in PEQ greenhouses at Headquarters for virus testing using specific antisera to various seed-transmitted viruses by enzyme-linked immunosorbent assay (ELISA), electron microscopy and Reverse-transcription-PCR.


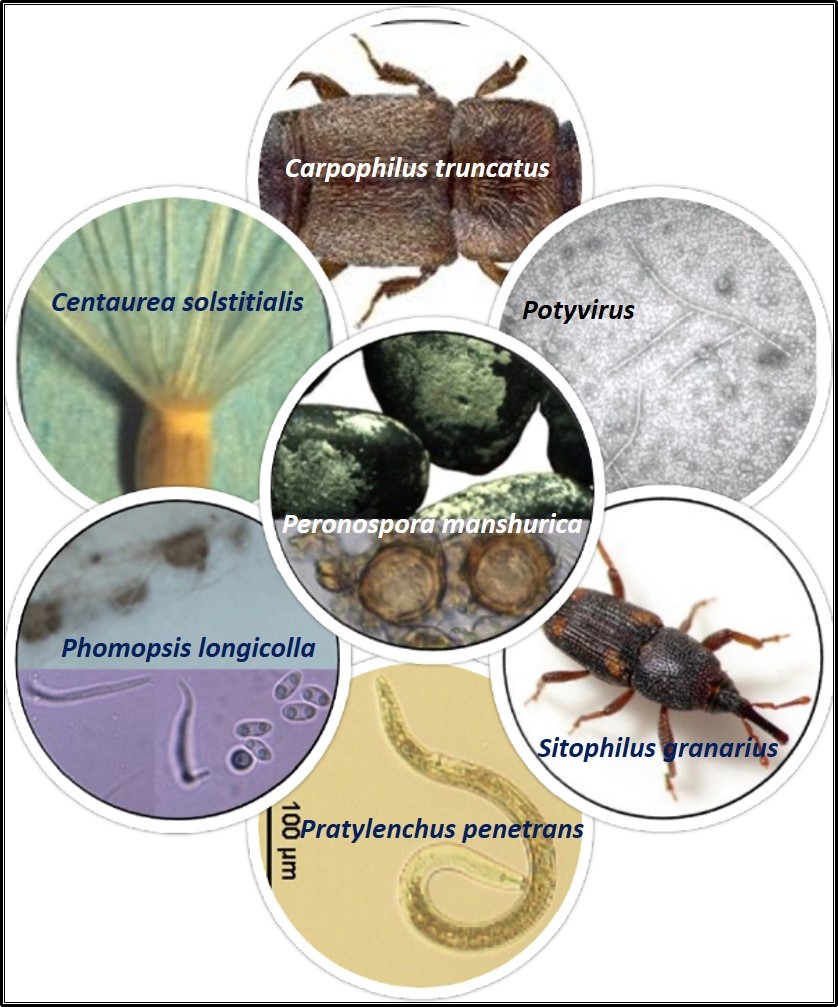
Exotic pests (not reported from India) intercepted in germplasm imported into India
Pests | Host | Source (Country) |
Insects | ||
Acanthoscelides desmanthi | Desmanthus spp. | Columbia |
A. obtectus | Cajanus cajan | Brazil, Colombia, Italy, Mexico, Nigeria, Peru, USA |
| Phaseolus vulgaris |
|
Anthonomus grandis | Gossypium sp. | USA |
| Hibiscus sp. |
|
Blapstinus histrichus | Carthamus tinctorius | USA |
Bootanomyia sp. | Eucalyptus sp. | Australia |
Bruchidius atrolineatus | Vigna unguiculata | Nigeria |
B. trifoli | Trifolium spp. | Egypt, Ethiopia, UK |
Bruchophagus gibbus | Medicago sativa | USA |
B. roddi | Trifolium pretense | USA |
Bruchus atomarius | Lens culinaris | Lebanon |
Bruchus dentipes | Vicia faba | Syria |
V. narbonensis | Afghanistan | |
B. ervi | Lens culinaris | Chile, Greece, Iran, Syria, Lebanon, Italy, Germany, Morocco, Turkey, Russian Federation |
B. nubilis | V. faba | Ukraine |
B. rufimanus | V. faba | Afghanistan |
B. tristis | Lathyrus sativus | Syria, Lebanon |
Callosobruchus subinnotatus | Vigna subterranea | Ghana |
C. rhodesianus | V. unguiculata | Nigeria |
Carpophilus truncatus | Zea mays | USA |
Ephestia elutella | T. aestivum | Italy |
Gymnetron plantaginis | Plantago lanceolata | Italy |
Oscinella frit | H. vulgare | Sweden |
Pachymerus lacerdae | Orbygnya phalerata | Brazil |
Quadrastichodella eucalypti | Eucalyptus camuldensis | Australia |
Sitophilus granarius | T. aestivum | USA |
Trogoderma variabile | T. aestivum | Australia |
Typhaea stercorea | Mangifera indica | Israel |
Viruses | ||
barley stripe mosaic virus | Hordeum vulgare | USA |
Triticum aestivum | USA | |
Zea mays | Philippines, USA | |
bean mild mosaic virus | Glycine max | Canada, Columbia |
Phaseolus vulgaris | USA | |
bean pod mottle virus | G. max | USA |
broad bean stain virus | Pisum sativum | Spain |
Vicia faba | ICARDA (Syria), Bulgaria | |
broad bean true mosaic virus | P. vulgaris | USA |
broad bean mottle virus | P. vulgaris | AVRDC (Taiwan) |
cherry leaf roll virus | G. max | AVRDC (Taiwan), Sri Lanka, Thailand, USA |
P. vulgaris | CIAT (Colombia) | |
cowpea mottle virus | Vigna unguiculata | Philippines |
V. subterranea | Ghana | |
cowpea severe mosaic virus | G. max | USA |
V. radiata | Australia | |
V. unguiculata | Belgium | |
high plains virus | Z. mays | USA |
maize chlorotic mottle virus | Z. mays | Puerto Rico, USA |
pea enation mosaic virus | G. max | Costa Rica |
P. vulgaris | Nepal | |
P. sativum | Spain, USA | |
V. faba | ICARDA (Syria) | |
peanut stunt virus | P. vulgaris | USA |
G. max | Costa Rica | |
pepino mosaic virus | Capsicum annuum | Republic of Korea |
Solanum lycopersicum | Netherlands, Thailand, USA | |
raspberry ring spot virus | G. max | AVRDC (Taiwan), Costa Rica, Sri Lanka, Thailand, USA |
tomato ring spot virus | G. max | AVRDC (Taiwan), Canada, Costa Rica, Sri Lanka, Thailand, USA |
wheat streak mosaic virus | T. aestivum | USA |
Z. mays | Philippines, Puerto Rico, South África, USA | |
Fungi | ||
Fusarium nivale | Triticum aestivum | Germany, Hungary, Italy, Mexico, Sweden, Turkey, UK and USA |
| Hordeum vulgare | Italy |
F. oxysporum f. sp. cucumerinum | Cucumis sativus | USA |
Diaporthe eres | Helianthus annuus | USA |
D. heliianthi | H. annuus | USA |
D. longicolla | H. annuus | USA |
Peronospora manshurica | G. max | Belgium, Brazil, Canada, Colombia, Costa Rica, Ghana, Hungary, Indonesia, Israel, Italy, Japan, Korea, Malaysia, Nepal, Papua New Guinea, Poland, Romania, Russia, Taiwan, Thailand, USA and Zimbabwe |
Uromyces beticola | Beta vulgaris | Belgium, Denmark, Holland, Hungary, Germany, The Netherlands, Sweden, UK, USA and SSR |
Nematodes | ||
Aphelenchoides arachidis | Arachis hypogoea | Nigeria |
A. fragariae | Fragariae spp | USA |
Ditylenchus destructor | Solanum tuberosum | Australia |
D. dipsaci | Humulus lupulus | Germany and USA |
Heterodera humuli | H. lupulus | Australia, Germany |
H. schachtii | Beta vulgaris | Denmark and Germany |
H. goettingiana | Pisum spp. | UK |
Pratylenchus penetrans | Malus domestica | Belgium, Italy, France, Netherlands, USA |
Pratylenchus penetrans | Pyrus communis | France, Belgium |
Rotylenchus minutus | Hypoxis hemerocallidea | Swaziland |
Scutellonema bradys | Diascorea spp. | USA |
Weeds | ||
Avena barbata | H. vulgare | Morocco |
A. sterilis | T. aestivum | Australia |
Bifora testiculata | H. vulgare | Lebanon |
Bromus secalinus | Cicer arietinum | Australia |
Centaurea maculosa | Coriandrum sativum | Russia |
C. melitensis | H. vulgare | Morocco |
C. solstitialis | C. sativum | Russia |
Cichorium pumilum | Trifolium alexandrinum | Uzbekistan |
Cichorium spinosum | T. alexandrinum | Uzbekistan |
Convolvulus erubescens | H. vulgare | Morocco |
Echinochloa crus-pavonis | Oryza sativa | China |
Echium plantagineum | H. vulgare | Morocco |
Galium tricornutum | C. sativum H. vulgare Lathyrus sativus | Lebanon, Uzbekistan |
Hordeum pusilum | L. sativus | Lebanon |
Ipomoea plebeia | Nigella sativa | Uzbekistan |
Salsola vermiculata | Lens culinaris | Canada |

Export Quarantine
- A total of 10,488 indigenous crops meant for export to other countries were processed for quarantine clearance, of which 276 samples were found infested/ infected and 193 samples were salvaged and 173 Phytosanitary Certificates were issued.
Seed Health Testing for Pest-Free Conservation
- A total of 45, 801 indigenous including 619 cryo-preserved samples and 212 samples for virus indexing were processed before conservation in National Genebank, of which 8272 samples were found infested/ infected/ contaminated with insects (4,723 samples), pathogens (1563 samples), nematodes (1670 samples) and weeds (316 samples) and 620 samples could not be salvaged, hence, rejected.
Awards/Honours
Dr V Celia Chalam:
- P. Misra & R.N. Pandey IPS Best Women Scientist Award (2023)for outstanding contributions in Plant Pathology by Indian Phytopathological Society, New Delhi.
- Dr RDVJ Prasada Rao Memorial Award 2012 for outstanding contributions in Diagnostics & Plant Biosecurity by Plant Protection Association of India, Hyderabad.
- Fellow, Indian Phytopathological Society for outstanding contribution to the science of plant pathology for the year 2019.
- Fellow Indian Society of Plant Genetic Resources for outstanding contribution to the science of plant genetic resources for the year 2011.
Dr Kavita Gupta:
- National FAW expert to validate and endorse the guidelines formulated for FAW management in Asia under their FAO funded project entitled “Coordinative Surveillance and Early warning for sustainable management of transboundary plant pest in Asia”
- Invited to conduct a Workshop on ‘In-depth Pest Risk Assessment of Prioritized Pests in Bangladesh’ as a regional resource person with NPPO Bangladesh and CABI-South Asia in Dhaka, Bangladesh from 17-20 June, 2023.
Dr MC Singh:
- 3rd ICAR Award in Inter-institutional competition of Hindi Essay writing in 2019.
- Councillor (North Zone) of Indian Society of Weed Science for 2019-2021.
Dr Jameel Akhtar:
- Dr Bap Reddy Award (2018-2020) of Plant Protection Association of India, Hyderabad.
- KC Mehta & Manoranjan Mitra Memorial Award (2023) of Indian Phytopathological Society, New Delhi.
- Golden Pen Award (2023) of Indian Society of Mycology and Plant Pathology, Udaipur.
- Scientist of the Year Award (2019) by Agricultural & Environmental Technology Development Society, U.S. Nagar, UK, India.
- Outstanding Scientist Award (2020) by VDGOOD Professional Association, Villupuram Tamil Nadu
- Zonal President (Delhi Zone) of Indian Phytopathological Society for the session 2023-24.
- Editor-in-Chief of Journal of Mycology and Plant Pathology for the years 2023-25.IPS Fellow (2019) by Indian Phytopathological Society, New Delhi.
- ISPRD Fellow (2022) by Indian Society of Pulses Research and Development, Kanpur.
- PPAI Fellow (2023) by Plant Protection Association of India, Hyderabad.
- 3rd Prize in ‘Hindi Kavita Path Pratiyogita’ at ICAR-NBPGR, New Delhi during Hindi Pakhwada on Sep. 28, 2019.
Dr BH Gawade:
- Councillor 2021-22 (Delhi zone) in Executive committee of Nematological Society of India, New Delhi.
- Fellow, Nematological Society of India, New Delhi in February 2024.
- Young Scientist Award’ from Society for Biotic and Environmental Research (SBER), Khowai, Tripura for contribution in the field of Plant Nematology and Plant Quarantine.
Dr Pardeep Kumar:
- Young Biotechnologist Award (2019) by Agricultural & Environmental Technology Development Society (AETDS), U.S. Nagar, UK, India.
- Young Scientist Award (2020) by Society of Krishi Vigyan, Sonarpur, Kolkota, India.
- Councillor 2023-24 (Delhi zone) in Executive committee of Indian Phytopathological Society, New Delhi.
- PPAI Fellow (2023) by Plant Protection Association of India, Hyderabad.
- Young Scientist Award (2023) by IIMT University, Meerut, Uttar Pradesh.
Ms Raj Kiran:
- Young Scientist Award (2021) by Agro Environmental Development Society (AEDS) Rampur, Uttar Pradesh.
- Young Scientist Award (2019) by Agricultural & Environmental Technology Development Society (AETDS), U.S. Nagar, UK, India.
Dr Pooja Kumari :
- Young Scientist Award (2021) in Plant Pathology by Agro Environmental Development Society (AEDS) Rampur, Uttar Pradesh.
- PR Verma Award (2021) Best Ph.D. thesis Award of Indian Society of Mycology and Plant Pathology, Udaipur.
- Young Scientist Award (2020) by Agricultural & Environmental Technology Development Society (AETDS) U.S. Nagar, Uttarakhand, India
Dr Sadhana Maurya:
- Bronze Medal in Chess Competition of ICAR Central Zone Sports Meet 2022 at Indian Institute of Soybean Research, Indore (M.P.) during 3-6 January, 2023.
- 2nd Prize in badminton doubles and mixed doubles Competition in ICAR Central Zone Sports Meet 2023 at Central Institute of Agricultural Engineering, Bhopal (M.P.) during 18-21 December, 2023.
Best Oral Presentation Awards
- Large scale phenotyping of pea germplasm for discovery of resistant sources against powdery mildew (Erysiphe polygoni) by A Kumar, BR Meena, K Tripathi, S Tripathi, Gaya Charan, VK Sharma, J Akhtar, A Kumar and VC Chalam during 1st National Conference on ‘Plant Genetic Resources Management’ organized by Indian Society of Plant Genetic Resources, New Delhi, India at NASC Complex, New Delhi, on November 22-24, 2022
- Seed is a vehicle for transboundary movement of fungal pathogens: A quarantine concern” by J Akhtar, P Kumar, R Kiran, BR Meena, Sadhana, AK Maurya and VC Chalam during National e-Conference on ‘Biotic stress management strategies for achieving crop production and climate resilience’ organized by the Society of Plant Protection Science, New Delhi at ICAR- NCIPM, New Delhi on May 19-21, 2022.
- Development of duplex PCR assay for simultaneous detection of Diaporthe phaseolorum and D. longicolla infecting soybean by A Tripathi, J Akhtar and VC Chalam during National e-Conference on ‘Biotic stress management strategies for achieving crop production and climate resilience’ organized by the Society of Plant Protection Science, New Delhi at ICAR-NCIPM, New Delhi on May 19-21, 2022.
- Development of multiplex PCR for simultaneous detection of three seed-borne fungal pathogens of rice by P Kumar, J Akhtar, R Kiran, BR Meena, Sadhana, S Kaushik, R Tiwari and VC Chalam during National e-Conference on ‘Biotic stress management strategies for achieving crop production and climate resilience’ organized by the Society of Plant Protection Science, New Delhi at ICAR-NCIPM, New Delhi on May 19-21, 2022.
- Status of seed-borne fungi in indigenous solanaceous vegetable germplasm in India by P Kumar, J Akhtar, R Kiran, BR Meena, Sadhana, V Gupta, S Pandey, SC Dubey and VC Chalam during International e-Conference on ‘Postharvest disease management and value addition of horticultural crops’ organized by the Department of Plant Pathology, ICAR-IARI, New Delhi on August 18 – 20, 2021.
- Managing the threats of plant nematodes during international exchange of vegetative propagules of horticultural crops’ by Gawade BH, Khan Z and V. Celia Chalam in International e-Conference on “Postharvest Disease Management and Value Addition of Horticultural Crops” 2021 during August 18-20 at ICAR-IARI, New Delhi organized by Indian Phytopathological Society, New Delhi in virtual mode.
- Conventional techniques of seed health testing: pivotal in disease free import and safe conservation of plant genetic resources in National Genebank India by J Akhtar, R Kiran, P Kumar, M Shekhar, Sadhana AK Maurya and SC Dubey during Technical Session 17 on International Trade and Biosecurity of 7th IPS International Conference on Phytopathology in achieving sustainable development goals’ organized at ICAR-IARI, New Delhi on Jan. 16-20, 2020.
- Status of seed-borne fungi in some indigenous millet germplasm in India by Raj Kiran, J Akhtar, P Kumar, Sadhana and SC Dubey during Online International Conference ‘PAAS-2020’ organized by Agricultural & Environmental Technology Development Society (AETDS), U.S. Nagar, UK, India on Oct. 4-6, 2020.
- Detection and identification of plant parasitic nematodes during international exchange of vegetative propagules and rooted germplasm by Gawade BH, Khan Z and Dubey SC during 7th International Conference on ‘Phytopathology in achieving sustainable development goals’ organized at ICAR-IARI on Jan. 16-20, 2020.
- Agro-morphological evaluation of okra wild species, Abelmoschus moschatus germplasm: A potential source of OYVMD and OELCD resistance by Gangopadhyay, KK, Pooja Kumari, Pragya, Rakesh Srivastava, Vinod Kumar and SP Singh Presented in National Conference on Biodiversity and Plant Genetic Resources conservation for future at University of Agriculture and Horticultural Sciences, Shivamogga, Karnataka, India on March 15-16, 2019.
- Characterization of aflatoxigenic Aspergillus flavus associated with aflatoxin B1 (AFB1) production of maize kernel in India” by Pooja Kumari, Robin Gogoi and Meena Shekhar presented during 7th International conference on “Phytopathology in Achieving UN Sustainable Development Goals” organised by Indian Phytopathological Society on January 16-20, 2020, ICAR-IARI, New Delhi, India.
- Screening for okra yellow vein mosaic disease resistance in wild okra (Abelmoschus moschatus ssp. moschatus) germplasm in India by Pooja Kumari on the occasion of International Web Conference prespective on Agricultural and Applied Sciences in COVID-19 Scenario organised by Agricultural & Environmental Technology Development Society (AETDS) U.S. Nagar, Uttarakhand, India on October 4-6, 2020.
- Molecular characterization of monopartite bhendi yellow vein mosaic virus infecting wild okra (Abelmoschus moschatus ssp. moschatus) in India by Pooja Kumari during 3rd International Conference on Global Initiatives in Agricultural, Forestry and Applied Sciences for Food Security, Environmental Safety and Sustainable Development (GIAFAS-2021)’’organised by Agricultural & Environmental Technology Development Society (AETDS) at Shri Guru Ram Rai University, Dehradun, Uttarakhand, India, held on October 17-18, 2021 by virtual mode.
Best/Outstanding Poster Awards
- Development of diagnostics for simultaneous detection of fungal pathogens, Peronspora manshurica and Diaporthe phaseeolorum infecting soybean by Aradhika Tripathi, Jameel Akhtar and V Celia Chalam during National Conference on ‘Plant Health for Food Security: Threats and Promises’ organized by Indian Phytopathological Society at ICAR-Indian Institute of Sugarcane Research, Lucknow on February 1-3, 2024.
- Pathogen interceptions in quarantine testing of imported planting materials for agricultural biosecurity by Jameel Akhtar, Bharat Raj Meena, Pardeep Kumar, Raj Kiran, Sadhana Maurya, Ashok Kumar Maurya and V Celia Chalam during National Symposium on ‘Seed Health and Disease Management for Agricultural Biosecurity & Annual Zonal (IPS – Delhi Zone) Meeting’ organized by Indian Phytopathological Society (Delhi Zone) at ICAR-National Bureau of Plant Genetic Resources, New Delhi on January 17, 2024.
- PCR based diagnostics for detection of fungal pathogen Tilletia barclayana causing kernel smut of rice by Aradhika Tripathi, Jameel Akhtar and V Celia Chalam during National Symposium on ‘Seed Health and Disease Management for Agricultural Biosecurity & Annual Zonal (IPS – Delhi Zone) Meeting’ organized by Indian Phytopathological Society (Delhi Zone) at ICAR-National Bureau of Plant Genetic Resources, New Delhi on January 17, 2024.
- Phenotyping of Brassica germplasm for identification of resistant sources against Sclerotinia sclerotiorum by Varun K, DK Kasyap, R Yadav, M Singh, AK Gupta, PD Meena and J Akhtar presented in National Symposium on ‘Recent trends in Phytopathology to address emerging challenges for achieving food security’ at ICAR-VPKAS, Almora on February 20-21, 2022.
Major Activities
- Inspection of introduced germplasm including transgenics for detection of pests
- Identification of intercepted pests
- Post-entry quarantine growing and inspection
- Salvaging of infested/ infected/ contaminated germplasm
- Issue of Phytosanitary Certificate for material intended for export
- Pest risk analysis
- Inspection of germplasm for detection of associated pests
- Eco-friendly salvaging of the infested/ infected/ contaminated germplasm
- Diagnostic protocols development for pests and diversity analysis
- Development of phytosanitary treatments
- Preparation of checklists of pests of various crops for risk analysis
- Germplasm evaluation for biotic stresses
- Assessment of prevalence of seed-transmitted viruses of legumes
- Survey for emerging pests and probable crop loss estimation
- DNA bar code Callosobruchus chinensisand maculatus: Cytochrome oxidase I gene based DNA barcode for C. chinensis (bp) 682 and C. maculatus (683) were developed.
- Quadruplex and qPCR assays: Quadruplex and qPCR assays were developed targeting the sequences of different genes such as Histone-3 for detection of longicolla and M. phaseolina. The markers DlHisF2&R2 and MpHisF1&R1 produced 265 and 309 bp amplicons for D. longicolla and M. phaseolina, respectively. Actin gene based marker DpActF1&R2 was developed for D. phaseolorum which provided 113 bp amplicon whereas, COX2 based marker PmCoxF2&R2 was developed for P. manshurica with amplified product of 152 bp. These markers proved highly specific and sensitive for detection of these pathogens up to 0.1 pg of template DNA using qPCR. Quadruplex PCR protocol was also developed by combining these specific markers which could distinguish all the targeted pathogens simultaneously in a single reaction.
- Multiplex PCR assay for simultaneous detection of Alternariabrassicicola, A. brassicae and Xanthomonas campestris campestris: Three sets of primers namely, Aba28sF and Aba28sR based on SSR marker for Alternaria brassicicola, ABC transporter (Atr1) gene-based primers, AbeABC1F and AbeABC1R for A. brassicae and rpf region-based primers namely rpfH_F and rpfH_R for X. campestris pv. campestris were developed. The specific bands of 586 bp for A. brassicae, 201 bp for A. brassicicola and 304 bp for X. campestris pv. campestris were obtained in multiplex PCR. The sensitivity of the primer pairs is 100 pg µl-1 of template DNA.
- Duplex PCR for simultaneous detection of Alternaria padwickiiand Bipolaris oryzae infecting rice: A set of primers namely BoSP7-F and BoSP7-R designed from oryzae ATCC44560 unplaced genomic scaffold scaffold_136 whereas ApEF-1F and ApEF-1R were designed from elongation factor 1 region of A. padwickii. The specific bands of 175 bp for A. padwickii and 325 bp for B. oryzae were obtained in duplex PCR. The detection sensitivity of the primer pairs with the diluted genomic DNA revealed that it could detect up to 0.1 ng µl-1 of template DNA of both the pathogens.
- Development of RT-PCR protocols for detection of viruses: Reverse-transcription – PCR protocols were developed for detection of Bean common mosaic necrosis virusand Peanut stunt virus (PSV). PSV is not reported from India.
- Development of Quintuplex RT-PCR protocols for detection of soybean viruses: Quintuplex RT-PCR protocol was optimised for the detection of viruses infecting soybeannamely, bean pod mottle virus (538 bp), cherry leaf roll virus (139 bp), raspberry ringspot virus (229 bp), tomato ringspot virus (298 bp) and soybean mosaic virus (409 bp) simultaneously.
- Tetraplex RT-PCR protocol for detection of maize viruses: Tetraplex RT-PCR protocol was optimised for the detection of maize infecting barley stripe mosaic virus (BSMV, 397bp), maize chlorotic mottle virus (MCMV, 182bp), maize dwarf mosaic virus (MDMV, 336bp) and wheat streak mosaic virus (WSMV, 948bp) simultaneously.
- Genetic diversity analysis of fungal pathogens using different molecular markers: Genetic diversity of fungal pathogens, namely, padwickii, Curvularia lunataand Fusarium spp. using SRAP markers, Bipolaris oryzae using URP and RAPD primers, B. sorghicola and C. capsici using URP and ISSR markers, C capsici and F. verticillioides using ISSR markers were analysed. Phylogenetic analysis among the isolates of fungal pathogens such as A. padwickii, Bipolaris spp., Curvularia spp., Exserohilum sp. using ITS markers and Fusarium spp. using ß-tubulin gene were done.
- Molecular characterization and phylogenic analysis of wheat seed gall nematode, Anguina tritici: Wheat seed gall nematode, Anguina tritici is one of the important nematodes of wheat (Triticum aestivum), which is seed borne in nature. The study was conducted on molecular characterization of A. tritici using gene sequences of different conserved regions such as Internal Transcribed Region (ITS), D2-D3 expansion region of 28s rRNA and phylogeny studies. Gene sequences of both the regions were PCR amplified using universal primers and corresponding size of ITS and D2-D3 region of 28s rRNA was found ~950 bp and ~750 bp, respectively. Both ITS and 28s rRNA gene products were sequenced and found identical to A. tritici populations (>99% similarity). Phylogeny trees were used to ascertain the relationships of it with other nematodes of Anguinidae family and revealed that A. tritici population studied was different from other seed gall forming and aerial nematodes, however, it was similar to other A. tritici populations.
- Thermal treatment against rice weevil: Thermal treatment i.e. 35, 40, 45, 50, 55 and 60±2 °C for a period of 1, 2 and 3 hours were evaluated against rice weevil, Sitophilus oryzae Cent percent mortality of S. oryzaeadult was observed at 45, 50, 55 and 60±1 °C for all time periods without affecting germination and vigour of wheat seeds.
- Evaluation of non-edible oils against chinensis: Hundred percent egg mortality was noticed in all non-edible oils of Pongamia glabra, Hydnocarpus wightiana, Madhuca longifolia, Callophyllum inophyllum, and Azadirachta indica compared to control. Similarly, all non-edible oils had ovipositional deterrence as compared to control. All non-edible oils caused hundred percent larval and adult mortality than control.
- Developed treatment for salvaging of nematode infested apple sapling: Root lesion nematode, Pratylenchus penetransnot reported from India was intercepted in apple (Malus domestica) rooted sapling imported from Belgium, Italy, France, Netherlands, USA. Root dip for 10 min in 0.25% formaldehyde solution was imparted and found effective. It eliminated nematode infection and hence recommended for apple bare rooted plants.
- Identified nematicidal properties of Soh-phlang, Flemingia procumbens for management of root-knot nematode: Nematicidal properties of Flemingia procumbens, an underutilized native crop species grown in the tribal tracts of Khasi and Jaintia hills of Meghalaya, India were identified for first time against root-knot nematode (RKN), Meloidogyne incognita. In vitro studies were conducted on second stage juveniles (J2s) of incognita using powder extract of small, medium and large size tubers. All the three types of powder extracts were found effective and caused varied mortality in J2s of M. incognitain the range of 88–95% at 100 mg/ml after 72 h exposure. Further studies upon fractional extraction showed methanol extract was effective in RKN control in vitro. This crop’s nematicidal potential can further be useful in RKN management under field conditions.
- Report on root-knot nematode incognita infestation on tuberous Vigna vexillata: Vigna vexillata, a wild species closely related to the cultivated cowpea (Vigna unguiculata (L.) Walp), was found with severe infestation of root-knot nematode (RKN), Meloidogyne incognita with heavily galled roots (galling index of 4.3) The soil population of J2s was also recorded well above the economic threshold level. The heavy root galling and presence of an extremely high population of J2 in soil (484-879 J2/ 200cc soil) indicates that M. incognita can be a potentially damaging pest to V. vexillata and can serve as a source of infestation to other hosts to be planted in same field. The present findings will awake the V. vexillata growers to consider growing V. vexillata under proper sanitation conditions. Soil samples from the proposed planting site should always be tested for the presence of nematode well before planting.
- Survey for emerging nematode problem in guava orchards of Tamil Nadu: The survey was conducted in and around Coimbatore and Dindigul districts of Tamil Nadu for emerging root knot nematode infecting guava crop. Root-knot nematode species was found similar to quarantine nematode pest Meloidogyne enterolobii.
- Ageratum conyzoides identified as a potential source of root-knot nematode infestation in cultivated fields: Infestation of the root-knot nematode, Meloidogyne javanica was observed on conyzoides which is commonly found weed in India. This weed found in the fields, bunds and along water channels. During survey, soil and root samples were collected for analyses of nematode infestation on this weed. Root galls observed on plant roots were counted and found in the galling index (GI) of 3-4 along with egg masses of RKN. All the soil and root samples collected from various sites contained M. javanica J2s and eggs. Population density of J2s in soil varied from 297-543/200cc soil whereas eggs obtained from the infested roots were 4657-6587 eggs/per root system. It was found that, A. conyzoides harbors RKN and its presence may affect RKN management using crop rotation. Strong weed management program needed for successful management of RKNs. Farmers advised to uproot the weed plants completely along with root system, especially those growing in the fields and along water channels to minimize further spread of nematode into new fields.
- Identification of source of resistance to root-knot nematode in agri-horticultural crops: Evaluated more than 4,000 accessions of cultivated and wild germplasm of various crops for resistance to root-knot nematode, Meloidogyne incognita and identified several resistant sources.
- Effect of wheat genotypes on the growth and development of weeds: The influence of wheat genotypes of different stature was studied on the growth and development of Phalaris minor, Chenopodium album and Melilotus indica was studied. The tall wheat genotype (plant height about 115 cm) exerted a strong suppressing effect on the weed intensity and height of these weeds.
Plant Quarantine Information System: In order to facilitate import quarantine of plant genetic resources, “Plant Quarantine Information System” has been developed, validated and being used for retrieval of various information pertaining to Import Quarantine.
User-friendly digitized identification keys for Bruchids: The end users, especially Directorate of Plant Protection, Quarantine and Storage and its 35 Plant Quarantine stations spread across the country and Entomologists across the world including those in various ICAR Institutes and SAUs has been using it for quick and reliable identification of bruchids of quarantine significance. The web version has >150 registered users within a span of 1 year of its upload.
Weed Risk Assessment system developed: Weed Risk Assessment is a question based scoring system, containing 49 questions about the species. The questions include details of the plant’s climatic preferences, biological attributes, re-production and dispersal methods. The WRA uses responses to the questions to generate a numerical score that is positively correlated with weediness. The plants which have score between 0-6 are non-weeds, 7-11 are common weeds and which have >12 score, are serious weeds.
Technologies Recognized by ICAR
- DNA Barcode, multiplex PCR and qPCR assay for diagnosis of pathogens infecting pulse crops to facilitate safe exchange and healthy conservation of germplasm developed by Aradhika Tripathi, SC Dubey, Anjali Rai, Jameel Akhtar and Pardeep Kumar in 2023 (ICAR-CS-NBPGR-Technology-2023-005).
- Multiplex PCR method for detection seed-borne pathogens of brassicas developed by Raj Kiran, Pardeep Kumar, Jameel Akhtar and SC Dubey in 2023 (ICAR-CS-NBPGR-Technology-2023-020).
- Technical input to ICAR and Department of Agriculture & Cooperation of Ministry of Agriculture on policy matters of quarantine including issues in EXIM, harmonization of phytosanitary standards with World Trade Organization (WTO) norms, etc.
- Teaching of post graduate students
- Training courses on biosecurity, quarantine techniques for diagnostics of pests, biosafety and related policy issues



Publications/Research Papers
Research Papers
- Akhtar J, B Singh, P Kumar, R Kiran, M Shekhar, Sadhana S, Pandey, S Lenka and SC Dubey (2020) Effect of stem gall disease on long-term germplasm preservation and quality seed production of coriander. Bhartiya Vaigyanik Evam Audyogik Anusandhan Patrika 28(2): 123-131.
- Akhtar J, B Singh, R Kiran, P Kumar, M Shekhar, Sadhana, S Pandey, S Lenka and SC Dubey (2020) Studies on infection indexing and distribution profiling of seed-borne fungi of sorghum germplasm in India for safe and healthy long-term conservation. Indian Journal of Plant Genetic Resources 23(3): 337-349.
- Akhtar J, P Kumar, R Kiran, BR Meena, A Ali, Sadhana and SC Dubey (2021) Bio-efficacy of essential oils in eco-friendly management of Tilletia barclayana causing kernel smut in rice. J Eco-friendly Agriculture 17(1): 162-165.
- Akhtar J, P Kumar, R Kiran, BR Meena, A Tripathi, Sadhana, S Pandey and SC Dubey (2023) First report of Diaporthe helianthi infecting safflower: A threat to its cultivation in India. J Phytopath. 171: 145-149.
- Akhtar J, P Kumar, R Kiran, BR Meena, Sadhana, V Gupta, S Pandey and SC Dubey (2023) First report of Diaporthe phaseolorum infecting Indian trumpet flower (Oroxylum indicum) from India. BioResources 18(2): 3101 – 3108.
- AkhtarJ, K Tripathi, M Akram, Naimuddin, US Rathore, A Kumar and VC Chalam (2022) Characterization of novel strain of Candidatus Phytoplasma aurantifolia infecting cowpea (Vigna unguiculata). Journal of Food Legumes 35(4): 251-259.
- Baite MS, BK Upadhyay and SC Dubey (2019) Development of a sequence-characterized amplified region marker for detection of Ascochyta rabiei causing Ascochyta blight in chickpea. Folia Microbiol. 65: 103-108.
- Bhanushree N, BS Tomar, J Akhtar and P Saha (2022) Identification of new resistant varieties to Phomopsis fruit rot of eggplant (Solanum melongena). Indian J Agri Sci. 92(9): 1143-1147.
- Bhanushree N, BS Tomar, J Akhtar; TK Behera; RK Ellur; RMV Sevanthi, S Jaiswal and P Saha (2023) Genetic analysis and QTL identification for Phomopsis blight resistance in eggplant. Plant Breeding 142: 700-710.
- Boopathi, T, S Mohankumar, Gayacharan, M Kalyanasundaram, SB Singh, R Aravindraj, B Preetha, K Sankari Meena, and K Chandrasekar (2019) Host-based genetic divergence in populations of an exotic spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae). European J. Entomol., 116: 221–228.
- Boopathi, T., SB Singh, SK Dutta, V Dayal, AR Singh, S Chowdhury, Y Ramakrishna, I Shakuntala, and K. Lalhruaipuii (2019). Biology, predatory potential, life table and field evaluation of the ladybird beetle, Propylea dissecta (Coleoptera: Coccinellidae) against Lipaphis erysimi (Hemiptera: Aphididae) on broccoli. Econ. Entomol. 113(1), 88-97.
- Chalam VC, Kavita Gupta, MC Singh, Z Khan, J Akhtar, BH Gawade, Pooja Kumari, Pardeep Kumar BR Meena, AK Maurya and DS Meena (2022) Role of plant quarantine in preventing entry of exotic pests. Indian Journal of Plant Genetic Resources 35(3): 141-146 DOI 10.5958/0976-1926.2022.00057.2
- Chalam VC and AK Maurya (2018) Role of quarantine in ensuring biosecurity against transboundary plant viruses. Agricultural Research Journal 55(4): 612-626.
- Chalam VC, Parakh DB and Maurya AK (2017) Role of viral diagnostics in quarantine for plant genetic resources and preparedness. Indian Journal of Plant Genetic Resources 30: 271-285
- Deepika DD, K Tripathi, GJ Abhishek, N Singh, R Sharma, VD Sharma, AK Maurya and VC Chalam (2023) Detection and identification of BCMV resistant germplasm in mungbean (Vigna radiata) using serological and molecular diagnostics. Indian Journal of Agricultural Sciences 93 (1): 98-101.
- Devindrappa, M., Kamra, A., Grover, B Gawade (2022). Nematicidal rhizobacteria with plant growth-promoting traits associated with tomato in root-knot infested polyhouses. Egyptian Journal of Biological Pest Control 32: 51. (2022) 32:51.
- Dubey SC, A Tripathi and S Indira (2019) Development of bio-agent based module for integrated management of sheath blight (Rhizoctonia solani) of rice. Indian J. Agril. Sci. 89: 663-669.
- Dubey SC, VD Sharma, VK Prajapati, J Akhtar and A Kandan (2022) Phenotypic variability, race profiling and molecular diversity analysis of India populations of Fusarium oxysporum sp. lentis causing lentil wilt. Folia Microbiologica 76(5): 757-775.
- Dutta, SK., J Layek, RS Akoijam, T Boopathi, VS Saha, SB Singh, Lungmuana and N Prakash (2019) Seaweed extract as natural priming agent for augmenting seed quality traits and yield in Capsicum frutescens J. Appl. Physiool., 31: 3803–3813.
- Dutta, SK., RS Akoijam, T Boopathi, S Saha, SB Singh, SK Das and A Yadav (2019) Mango under high density planting: A case study from North East India. Indian J Horti 76(2): 358-363.
- Esan AM, Khan Z, Kiran R, Omolekan TO, Aremu KA, and Adeyemi HRY (2021). Ameliorative Effects of Pseudomonas fluorescence strains on growth and antioxidant potential of okra (Abelmoschus esculentus) plant under nematode infection. European Journal of Biology and Biotechnology 2(3):50-56.
- Gautam D, N Ranjan, AB Gaikwad, KV Bhat, B Mondal, J Akhtar, G Singh, AM Iquebal, B Tiwari and S Archak (2020) Identification of new source of resistance against downy mildew disease of cucumber from a selected set of cucumber germplasm and its wild relatives. Indian J Genetics and Plant Breeding 80(4): 427-431.
- Gawade BH, A Pandey, Z Khan, S Niveditha, and SC Dubey (2019). First report on nematicidal properties of Flemingia procumbensagainst root-knot nematode Meloidogyne incognita. Indian Phytopath. 72: 551-553.
- Gawade BH, Chaturvedi S, Apsara N, Khan Z, Pandey CD, Gangopadhyay KK, Dubey SC and Chalam VC (2022). Evaluation of brinjal germplasm against root-knot nematode, Meloidogyne incognita. Indian Phytopathology 75: 449-456.
- Gawade BH, Chaturvedi S, Z Khan, CD Pandey, KK Gangopadhyay, SC Dubey and VC Chalam (2022) Evaluation of brinjal germplasm against root-knot nematode, Meloidogyne incognita. Indian Phytopathology 75: 449-456.
- Gawade BH, Priyanka, Z Khan, P Kumar and VC Chalam (2022) Molecular characterization of wheat seed gall nematode, Anguina tritici. Indian J Nematol 52(1): 28-34.
- Gawade BH, Z Khan, P Hollajer and SC Dubey (2019) Detection and identification of root-knot nematode, Meloidogyne javanicaon Ageratum Indian J Nematol 49(1): 113-115.
- Ghorai PS, S Biswas, TJ Purakayastha, N Ahmed, TK Das, R Prasanna, BH Gawade, K Bhattacharyya, K Sinha, P Singh, S Das (2023). Indicators of soil quality and crop productivity assessment at a long-term experiment site in the lower Indo-Gangetic plains. Soil Use and Management 39 (1): 503-520.
- Gowda BC, A Kumar, SK Lal, J Akhtar, GP Mishra, SK Tiwari, Ravindra Kumar and S Javeria (2023) Evaluation of biocontrol agents for management of fruit rot and its effect on seed quality in chilli. Indian J Horticulture 79 (4): 450-457.
- Gupta K and Sankaran KV (2021) Forest biosecurity systems and processes: An Indian perspective. Frontiers of Forests and Global Change 4: 699950.
- Gupta K, S Bhalla, SP Singh and DS Meena (2018) Seed beetles intercepted in imported legume germplasm from 2001- 2015. Legume Research. 41: 629-635
- Gupta K, Sankaran KV and PS Kumar (2023) Exchange of biological control genetic resources in India: prospects and constraints for access and benefit sharing. BioControl68: 281-289.
- Gupta K, SP Singh, S Bhalla and DS Meena (2021) Interception of insect pests using x-ray radiography during quarantine of plant genetic resources. Indian Journal of Plant Genetic Resources. 34(1): 34-44.
- Gupta K, SP Singh, S Bhalla and DS Meena (2021) Interception of insect pests using X-Ray radiography during quarantine of plant genetic resources. Indian Journal of Plant Genetic Resources 34(1): 43–53.
- Hemavati Ranebennur, K Rawat., A Rao, P Kumari, VC Chalam, N Meshram and GP Rao (2022) Transmission efficiency of a ‘Candidatus Phytoplasma australasia‘(16SrII-D) related strain associated with sesame phyllody by dodder, grafting and leafhoppers. European Journal of Plant Pathology 164: 193-208,
- Hiremani NS and SC Dubey (2018) Race profiling of Fusarium oxysporum sp. lentis causing wilt in lentil. Crop Protection 108: 23-30.
- Hiremani NS and SC Dubey (2019) Genetic diversity of Fusarium oxysporum sp. lentis populations causing wilt of lentil in India. Indian Phytopath. https://doi.org/ 10.1007/s42360-019-00126-9.
- Holajjer P, BH Gawade, Z Khan and N Sivaraj (2022) Prediction of potential geographic distribution of exotic nematode, Aphelenchoides fragariae in India based on MaxEnt ecological niche modelling. Indian Journal of Plant Protection. 50(1): 51-55.
- Jadav S, A Kumar, SK Lal, J Akhtar, M Aski, GP Mishra, and S Javeria (2021) Development of SCAR marker or quick detection of Fusarium oxysporum sp. lentis in lentil (Lens culinaris). Indian J Agril Sci 92(2): 134 – 136
- Khan Z, K Tripathi, BH Gawade, VC Chalam (2022). Report on severe infestation of root-knot nematode, Meloidogyne incognita on tuberous Vigna vexillata (L.) Pantnagar Journal of Research 20 (1):53-57.
- Khan Z, Tripathi K, Gayacharan, Bharat BH, Chalam CV (2022). Observation on severe infestation of root-knot nematode, Meloidogyne incognita on tuberous Vigna vexillata. Pantnagar Journal of Research 20: 53-57.
- Kiran R, A Kandan, P Kumar, D Singh, J Akhtar, B Singh, SC Dubey (2019) Development of a specific primers from rpf gene sequence for molecular detection of Xanthomonas campestris pv. campestris. J. Environ. 41:105-110
- Kiran R, P Kumar, J Akhtar, K Nair and SC Dubey (2022) Development of multiplex PCR assay for detection of Alternaria brassicae, Alternaria brassicicola and Xanthomonas campestris pv. campestris. Archives of Microbiology 204: 204-224
- Koulagi R, S Banerjee, BH Gawade, AK Singh, PK Jain, Shelly Praveen, K Subramaniam and Anil Sirohi (2020) Host-delivered RNA interference in tomato for mediating resistance against Meloidogyne incognitaand Tomato leaf curl virus. Plant Cell, Tissue and Organ Culture 143, 345–361.
- Kumar N, SC Dubey, P Kumar and SMP Khurana (2019) Fusarium solani causing stem rot and wilt of lucky bamboo (Dracaena sanderiana) in India- first record. Indian Phytopath. 72: 367-371
- Kumar P, J Akhtar, A Kandan, B Singh, R Kiran, K Nair, SC Dubey (2018) Efficacy of URP and ISSR markers to determine diversity of indigenous and exotic isolates of Curvularia lunata. Indian Phytopathology 71: 235-242.
- Kumar P, J Akhtar, R Kiran, BR Meena, Sadhana and VC Chalam (2023) Phylogenetic analysis and species level identification of seed-borne Bipolaris, Exserohilum and Curvularia. Indian Phytopathology 76: 115–121
- Kumar P, SC Dubey, J Akhtar, R Kiran, K Nair and H Bhati (2020) Genetic diversity and population structure analysis of Colletotrichum capsici isolates using SRAP and URP markers. Indian Journal of Plant Protection 48(1&2): 64-73
- Kumar, Virender, Kumawat, Surbhi, Bisht Ashita. SM ShivaRaj, Padalkar, Gunashri, Goyal Vinod, Zargar sajad, Gupta Sanjay, Kumawat Giriraj, VC Chalam, Ratnaparkhe Milind, Gill Balwinder, Jean Martine, Patil Gunvant , Vuong Tri, Rajcan Istvan, Deshmukh Rupesh, Belzile Francois, Sharma Tilak, Nguyen Henry and Sonah Humira (2021) Breaking the negative correlation between seed oil and For Peer Review Only protein content: a long-standing challenge for soybean improvement. Critical Reviews in Plant Sciences 40 (5): 398-421.
- Kumari P, R Gogoi, N Srinivasa and M Shekhar. (2021) Characterization of aflatoxigenic Aspergillus flavus associated with aflatoxin B1 (AFB1) production in maize kernel in India. Indian Phytopathology 74: 103-112.
- Kumari P, Singh SP, Gangopadhyay KK, ChalamVC, Dubey SC and Srinivasa N (2021). Screening of wild okra (Abelmoschus moschatus) germplasm for okra yellow vein mosaic disease resistance in India. Indian J Agril Sci 91(7): 1010–1014.
- Kumari P, SP Singh, KK Gangopadhyay, VC Chalam, CD Pandey and SK Yadav (2022) Standardization of artificial screening technique for okra enation leaf curl disease resistance in wild okra (Abelmoschus moschatus Moschatus) germplasm. Indian Journal of Agricultural Sciences 92 (10): 1214–1218.
- Kumari P, SP Singh, KK Gangopadhyay, VC Chalam, SC Dubey and Pragya Ranjan (2021). Screening for okra enation leaf curl disease resistance in wild okra (Abelmoschus moschatus moschatus) germplasm in India. Indian J Agril Sci 91 (10): 1487–94.
- Kumari P, SP Singh, KK Gangopadhyay, VC Chalam, YB Basavaraj, V Venkataravanappa and A Kumar (2022) Molecular characterization of monopartite bhendi (Abelmoschus moschatus) yellow vein mosaic virus and screening of wild okra. Indian Journal of Agricultural Sciences 92 (11): 1369–1374.
- Mehta BK, R Chhabra, V Muthusamy, UZ Rajkumar, A Baveja, HS Chauhan, NR Prakash, VC Chalam, AK Singh and F Hossain (2021) Expression analysis of β‑carotene hydroxylase1 and opaque2 genes governing accumulation of provitamin‑A, lysine and tryptophan during kernel development in biofortifed sweet corn. 3Biotech 11: 325.
- Nagaraju, D.K., M.C. Singh, O.P. Verma and Ravi Prakash (2021). Interception of non-indigenous weed seeds in lentil and lentil husk shipments imported from Australia, Canada, U.S.A., and Sri Lanka to India. Indian Journal of Weed Science. 53(4): 417-420.
- Nishmitha K, R Singh, SC Dubey, J Akhtar, A. Tripathi and D Kamil (2023) Expression profiling and characterization of key RGA involved in lentil Fusarium wilt Race 5 resistance. World J Microbiol Biotech 39: 306.
- Nishmitha K, R Singh, SC Dubey, J Akthar, Tripathi K and D Kamil (2023) Resistance screening and in-silico characterization of cloned novel RGA from multi race resistant lentil germplasm against Fusarium wilt (Fusarium oxysporum sp. lentis). Front. Plant Sci. 14:1147220.
- Padhi SR, A Bartwal, R John, K Tripathi, Kavita Gupta, DP Wankhede, GP Mishra, S Kumar, S Archak and R Bhardwaj (2022) Evaluation and Multivariate Analysis of Cowpea [Vigna unguiculata (L.) Walp] Germplasm for Selected Nutrients—Mining for Nutri-Dense Accessions. Sustain. Food Syst. 6:888041. doi: 10.3389/fsufs.2022.888041
- Padhi SR, R John, A Bartwal, K Tripathi, Kavita Gupta, DP Wankhede, GP Mishra, S Kumar, JC Rana, A Riar and R Bhardwaj (2022) Development and optimization of NIRS prediction models for simultaneous multi-trait assessment in diverse cowpea germplasm. Nutr. 9:1001551.
- Panigrahi SK, K Tripathi and Kavita Gupta (2021) Screening of black gram (Vigna mungo) and its crop wild relatives against Callosobruchus maculatus (Fab.) and correlation of resistance with seed physical parameters. Indian J. Plant Genet. Resour. 34(3): 455–459 https://DOI 10.5958/0976-1926.2021.00039.5
- Panigrahi SK, K Tripathi, R Singh, R Kumar, P Sanghamitra, DP Wankhede, N Singh, Dubey KKD and Kavita Gupta. (2022) Evaluation of black gram (Vigna mungo) genepool against Callosobruchus maculatus and diversity analysis inter se. The Indian Journal of Agricultural Sciences, 92(7), 915–919.
- Parameswari B, B Bajaru, L. Karthikaiselvi, N. Sivaraj, S. K. Mangrauthia, S. Nagalakshmi, Prasanna H, M. Srinivas, VC Chalam and K. Anitha (2023) First report of the association of Zygocactus Virus X with dragon fruit (Hylocereus ) plants from Telangana, India. Plant Disease 107: 1249.
- Parameswari B, B Bhaskar, Karthikaiselvi, N Sivaraj, P Pranusha, P Brahmi, SK Mangrauthia, L Saravanan, VC Chalam and K Anitha (2022) Interception of Bean common mosaic virus in bambara groundnut accessions imported from Ghana through reverse transcription polymerase chain reaction (RT-PCR). Indian Journal of Plant Protection 50: 2&3: 80-85
- Prasad NHP, A Kumar, SK Lal, P Shah, J Akhtar and SK Jha (2022) Assessment of relative performance of different genotypes of brinjal against fruit rot infection by artificial inoculation of Phomopsis vexans. The Pharma Innov. J 11(11): 221-224.
- Rajput LS, S Kumar, H Bhati, K Nair, J Akhtar, P Kumar and SC Dubey (2021) Morphological and cultural characterization of quarantine concerned Phomopsis associated with oilseed crops. J Mycol Pl Pathol 51(1): 48-58
- Rajput LS, S Kumar, H Bhati, K Nair, J Akhtar, P Kumar and SC Dubey (2021) Diversity assessment of indigenous and exotic Diaporthe species associated with various crops using ISSR, URP and SRAP markers. Indian Phytopath 74(3): 615-624
- Ranebennur H, K Kirdat, B Tiwarekar, K Rawat, VC Chalam, AU Solanke, R Yadav, K Singh, S Sathe, A Yadav and GP Rao (2022) Draft genome sequence of ‘Candidatus Phytoplasma australasia’, strain SS02 associated with sesame phyllody disease. 3 Biotech 12:107.
- Saabale PR, SC Dubey, P Kumari and TR Sharma (2018) Analysis of differential transcript expression in chickpea during compatible and incompatible interactions with Fusarium oxysporum sp. ciceris Race 4. 3Biotech 8: 111,
- Singh B, J Akhtar, A Kandan, P Kumar, D Chand, AK Maurya, PC Agarwal and SC Dubey (2018) Risk of pathogens associated with plant germplasm imported into India from various countries. Indian Phytopathology 72:91-102
- Singh K, J Akhtar, N Shekhawat, VS Meena, A Gupta, BR Meena, R Yadav, V Gupta and A Kumar (2022) Identification of new source of resistance against white rust. J Oilseed Brassica 13 (2): 100-104.
- Singh MC, BS Phogat and VC Chalam (2022) Growth and yield analysis under amaranth based inter-cropping system. International Journal of Tropical Agriculture 40(3-4): 419-422
- Singh MC, VC Chalam, D Singh, S Kumar and S Gnansambandhan (2022) Risk associated with the weed seeds in imported grain. Indian Journal of Weed Science 54(4): 370-375.
- Singh S, S Sharma, A Ali, A Kandan, P Kumar and J Akhtar (2021) Morpho-molecular characterization of Bipolaris and Exserohilum infecting various agricultural crops. Int. J. Agric. App. Sci. 2(1):110-117
- Singh SP, Kumari P, Gangopadhyay KK and Dubey SC (2021). Screening of wild okra against okra leafhopper insect, Amarasca bigutella bigutella. Indian J Agril Sci 91(8): 1199–1204.
- Singh, Dhruv, S. Gnansambandhan, M.C. Singh and Ravi Prakash (2020). Host-specificity in biological control of weeds using insects.J. Sci. Environ. Technol. 9: 995-1005
- Singh, H, D Maithani, MC Singh and Madhu B. Priyadarshi (2020). Study of antimicrobial activity of some selected weed species.J. Sci. Environ. Technol. 9: 61-65.
- Singh, H, D Maithani, MC Singh and MB Priyadarshi (2020). Study of antimicrobial activity of some selected weed species. J. Sci. Environ. Tech. 9 (1): 61-65
- Singh, MC, BS Phogat and I Singh (2019). Effect of different wheat genotypes on weed species. International Journal of Science, Environment and Technology. 8: 247-252
- Singh, MC, BS Phogat and VC Chalam (2022). Growth and Yield Analysis under Amaranth based Inter-cropping System. International Journal of Tropical Agriculture. 40(3-4): 419-422.
- Singh, MC, BS Phogat, I Singh, YS Rathi, and SC Dubey (2019). Study of weeds problem in wheat germplasm grown at NBPGR. J. Sci. Environ. Tech. 8 (2): 411 – 415
- Singh, MC, Madhu B. Priyadarshi and H Singh (2020). Preventing exotic noxious weeds through weed risk analysis. J. Sci. Environ. Technol.9: 48-54.
- Singh, SP, S Bhalla, K Gupta, DS Meena and SC Dubey (2019). Efficacy of thermal treatments against rice weevil, Sitophilus oryzae. Indian J. Agricul. Sci., 59(8): 1359-61
- Sivaraj N, SR Pandravada, A Agrawal, V Kamala, VC Chalam and K Anitha (2022) Application of geographical information system for PGR management. Indian Journal of Plant Genetic Resources 35(3): 141–146.
- Srivastava V, DK Nerwal, A Kandan, J Akhtar, N Sharma, R Kiran, S Bansal and A Agrawal (2020) Management of microbial contaminants in the In Vitro Gene Bank: a case study of taro [Colocasia esculenta (L.) Schott]. In Vitro Cellular & Developmental Biology – Plant 57:152–163
- Tiwari B, R Kiran, P Kumar, A Kumar, Sadhana, J Akhtar and SC Dubey (2021) Lasiodiplodia theobromae, a potential post-harvest threat to agri-horticultural crops and its morpho-molecular diversity. Indian J Pl Protec 49(2): 125-130
- Tripathi A, A Rai, SC Dubey, J Akhtar and P Kumar (2021) DNA barcode, multiplex PCR and qPCR assay for diagnosis of pathogens infecting pulse crops to facilitate safe exchange and healthy conservation of germplasm. Archives of Microbiology 203: 2575-2589
- Tripathi A, Dubey SC, J Akhtar and P Kumar (2022) Development of PCR-based assays to diagnose the major fungal pathogens infecting pulse crops, potential for germplasm health certification and quarantine processing. World Journal of Microbiology and Biotechnology 39: 74.
- Tripathi A, J Akhtar, Kalaiponmani, SC Dubey and VC Chalam (2023) Quadruplex and q-PCR based diagnostic assay to delineate the major quarantine and other seed-borne fungal pathogens of soybean. World Journal of Microbiology and Biotechnology 39: 233.
- Tripathi A, J Akhtar, P Kumar, Kalaiponmani and VC Chalam (2023) Diagnostic assay for molecular detection of Bipolaris maydis and Stenocarpella maydis for safe exchange and long-term conservation of maize germplasm. Physiol Mol Plant Pathol 128: 102165.
- Yadav DL, P Jaisani, RN Pandey and VC Chalam (2021) Detection and molecular characterization of Bean Common mosaic virus in mungbean.. International Journal of Chemical Studies 9 (1): 2996-3001.
Projects
Inhouse Projects
Sub-Project No. | Title | PI/ Co-PI and Associates |
1 | Detection, identification and phytosanitary treatments for fungi and bacteria in quarantine and supportive research | Jameel Akhtar, Pardeep Kumar, AK Maurya, Smita Lenka and Sadhana Maurya |
2 | Detection, identification and phytosanitary treatments for viruses, viroids and phytoplasma in quarantine and supportive research | V Celia Chalam, Pooja Kumari, AK Maurya and Smita Lenka |
3 | Detection, identification and phytosanitary treatments for insect and mite pests in quarantine and supportive research | Kavita Gupta and DS Meena |
4 | Detection, identification and phytosanitary treatments for nematode pests and weeds in quarantine and supportive research | Mool Chand Singh, Zakaullah Khan and BH Gawade, DS Meena |
5 | Seed health testing for pest-free conservation of indigenous germplasm and virus indexing of in-vitro cultures | Bharat H Gawade, V Celia Chalam, Kavita Gupta, MC Singh, Zakaullah Khan, Jameel Akhtar, Pardeep Kumar, Pooja Kumari, Sushil Pandey, Sandhya Gupta, AK Maurya, DS Meena, Smita Lenka and Sadhana Maurya |
Externally Funded Projects
Sl. No. | Project Title | Funding Agency | Principle Investigator | Date of Start | Date of Completion | Budget (Lakhs) | Project Code |
01 | Development of DNA barcode and multiplex PCR based diagnostics for detection of nationally important seed borne fungal pathogens of major pulse crops for safe exchange and conservation. | DBT | S C Dubey | April 2018 | March 2021 | 65.55 | 1009256 |
02 | Mainstreaming of Sesame germplasm for productivity enhancement through genomics assisted core development and trait discovery (Subproject-3 Identification of Biotic Stress (Phyllody & Dry Root Rot) Tolerant Sesame Genotypes; Component-4 Dry Root Rot | DBT | Pardeep Kumar | February 2020 | February 2025 | 45.57 | 1012160 |
03 | Mainstreaming of Sesame germplasm for productivity enhancement through genomics assisted core development and trait discovery Subproject-3 Identification of Biotic Stress (Phyllody & Dry Root Rot) Tolerant Sesame Genotypes; Component-1 Phyllody | DBT | V Celia Chalam | February 2020 | February 2025 | 60.27 | 1012157 |
04 | National containment/quarantine facility for transgenic planting material (component A) | DBT | V Celia Chalam | April 2013 | Sept 27, 2020 | 117.42 | 1001489 |
05 | Leveraging genetic resources for accelerated genetic improvement of linseed using comprehensive genomics and phenotyping approaches (Sub-Project 4: Evaluation of linseed germplasm for major biotic stresses (Alternaria blight and Linseed bud fly), Component 6.) | DBT | Kavita Gupta | April 2020 | March 2025 | 85.74 | 1012163 |
06 | National Programme for Quarantine and GM Diagnostics of Genetically Engineered Plant Material (Component 1) | DBT | V Celia Chalam | Sep 2020 | Sept 2025 | 470.328 | 1012553 |
Electron beam (EB) irradiation has potential for disinfestation of seeds infested with different stages of Callasobruchus chinensis and C. maculatus. However, the mortality is caused at higher doses but sterility is caused at lower doses. Dose of 340 Gy at 500 Kev has been found an effective dose with no significant effect on seed quality parameters of black gram and cowpea. Dose dependent effects of irradiation were observed on insect and seed parameters. Sterility in adults is caused at a very low dose (25 Gy), however, adult mortality occurs at higher doses. Gamma irradiation with doses upto 200 Gy resulted in increase in vigour index in cowpea seeds.
Study of biological control of invasive plant species and Indian natural enemies (ICAR-CAB International)Component B: Hedychium spp. complex (H. gardneriarum, H. flavescens, H. coronarium): Two surveys were undertaken in Sikkim areas and collected plant materials as well as infected/ infested samples were studied for their potential as natural enemies under controlled conditions at ICAR-NBPGR. Insect specimens submitted to National Pusa Collection at Division of Entomology, IARI for identification and pathogens submitted to Herbarium Cryptogamae Indiae Orientalis (HCIO) at Division of Plant Pathology, IARI and material sent to CABI, UK under MTA.
Race profiling and diversity analysis of Fusarium oxysporumf. sp. Lentiscausing lentil wilt in India (DST Funded)Lentil wilt (Fusarium oxysporum f. sp. lentis (Fol)) is one of the important diseases worldwide including India. Two hundred and thirty-five isolates of the pathogen collected from different parts of India showed substantial variations in respect of morphological characters such as colony texture, pattern, pigmentation, growth rate, etc. The Fol populations (70 representative isolates) were grouped into 7 races on the basis of virulence pattern obtained on 10 differential genotypes and distribution pattern of races in the country was determined. The genetic diversity was analyzed using molecular markers namely, random amplified polymorphic DNA (RAPD), universal rice primers (URPs), inter simple sequence repeats (ISSR) and sequence related amplified polymorphism (SRAP). The populations from northern and central regions of India were appears distinct. The sequences of rDNA ITS and TEF-1a genes of the representative isolates were analyzed. Phylogenetic analysis indicating that the molecular groups partially correspond to the lentil growing regions of the isolates and races of the pathogen.
Development of DNA barcode and multiplex PCR based diagnostics for detection of nationally important seed borne fungal pathogens of major pulse crops for safe exchange and conservation (DBT Funded)Internal Transcribed Spacer (ITS), Translation elongation factor (TEF-a), ß-tubulin, Cytochrome c oxidase (COX), Large subunit of the rRNA (LSU) and Small subunit of the rRNA (SSU) genomic regions proved to be suitable genes for characterization as well as proved suitable for species identification of the pathogens namely, Rhizoctonia solani, Macrophomina phaseolina (R. bataticola), Ascochyta rabiei, Alternaria alternata, A. tunuissima, Fusarium oxysporum f. sp. ciceris, Sclerotium rolfsii, Sclerotinia sclerotiorum, Pseudocercospora cruenta and Cercospora canescens. Universal internal transcribed spacer (ITS) region based DNA barcodes were developed for identification of A. alternata, A. tenuissima, A. rabiei, F. oxysporum f. sp. ciceris, M. phaseolina, R. solani, S. sclerotiorum and C. canescens and submitted to the Barcode of Life Data System (BOLD). The specific, highly sensitive and reliable conventional and real-time PCR assays were also developed for diagnosis of the pathogens namely, A. alternata, R. solani and F. oxysporum f. sp. ciceris associated with the pulse crops. Universal internal transcribed spacer (ITS) region-based markers namely, BAA2aF & BAA2aR for A. alternata and BRS17cF & BRS17cR for R. solani were developed and amplified 400 bp and 200bp species specific amplicons, respectively. Whereas, COX II region based marker FOCox1F & FOCox3R was developed for F. oxysporum f. sp. ciceris with amplicon size of 150 bp. All the markers proved to be highly specific and sensitive and are able to detect up to 0.0001 ng template DNA using qPCR.
National containment/ quarantine facility for transgenic planting material (DBT-funded)With the approval of RCGM, 3,554 samples of imported transgenic planting material comprising Arabidopsis thaliana (64), Brassica napus (93), Eucalyptus sp. (1,585), Glycine max (8), Gossypium hirsutum (134), Manihot esculenta (512), Nicotiana tabacum (2), Oryza sativa (926) and Zea mays (230) were processed for quarantine clearance. Important pests intercepted include seven fungi and infected samples were salvaged by giving fungicidal treatment. Seed samples belonging to different crops were grown in the containment facility for ~45 days for detection of seed-transmitted pests not detectable in the laboratory tests. Also, 28 post-entry quarantine inspections of different crops were undertaken at the indenter’s site. Thirteen viruses were detected in corn and soybean including nine viruses not reported from India viz., Barley stripe mosaic virus, Bean mild mosaic virus, Cherry leaf roll virus, Cowpea severe mosaic virus, High plains virus, Maize chlorotic mottle virus, Raspberry ringspot virus, Tomato ringspot virus and Wheat streak mosaic virus and one virus Arabis mosaic virus is not known to occur on the host and with limited distribution in India.
All the imported transgenic lines were tested to ensure the absence of embryogenesis deactivator gene by PCR with primers specific to the cre-lox system. Plasmid cloned with cre sequence was used as positive control. In PCR amplification of cre sequence, the amplicon of 1031bp size was amplified only in positive plasmid sample while, no amplicon of corresponding size was observed in any of these transgenic samples ensuring the absence of embryogenesis deactivator gene. The samples were also tested for specific transgenic elements using PCR/ Real-time PCR based GM diagnostics. A total of 58 samples comprising Arabidopsis thaliana (41) and Oryza sativa (17) were exported and two Phytosanitary Certificates were issued.
National Programme for Quarantine and GM Diagnostics of Genetically Engineered Plant Material (Component 1) (DBT-funded)With the approval of RCGM, 161 samples of imported transgenic planting material comprising Oryza Sativa (19), Arabidopsis thaliana (72), Zea mays (3), Cotton (59), Medigago truncatula (7) and Soybean (1) were processed for quarantine clearance. Important pests intercepted Diaporthe phaseolorum /D. longicolla and infected samples were salvaged by giving fungicidal treatment. Seed samples belonging to different crops were grown in the containment facility for ~45 days for detection of seed-transmitted pests not detectable in the laboratory tests. Also, 8 post-entry quarantine inspections of different crops were undertaken at the indenter’s site.
Specific reverse-transcription polymerase chain reaction (RT-PCR) protocols were developed for detection of bean pod mottle virus (BPMV), cherry leaf roll virus (CLRV), raspberry ringspot virus (RpRSV), tomato ringspot virus (ToRSV) and soybean mosaic virus (SMV) arabis mosaic virus (ArMV), grapevine fanleaf virus (GFLV) and bean yellow mosaic virus (BYMV) infecting soybean and barley stripe mosaic virus (BSMV), maize chlorotic mottle virus (MCMV), maize dwarf mosaic virus (MDMV) and wheat streak mosaic virus (WSMV) infecting maize using coat protein gene specific primers. Soil samples were collected from guava orchard, Ayakudy, Tamil Nadu and optimised PCR protocol for the detection of Meloidogyne enterolobii using universal ITS primers (403bp).
Duplex PCR assay was developed for simultaneous detection of Bipolaris maydis and Stenocarpella maydis infecting maize using a set of highly specific and sensitive primer sets, BmayITS1F&5R specific to B. maydis derived from ITS region and DMKB4F&4R specific to S. maydis derived from kilbournase gene was developed for simultaneous detection of both the fungal pathogens. Another duplex PCR assay was developed for simultaneous detection of Diaporthe phaseolorum and Peronospora. manshurica infecting soybean using a set of highly specific and sensitive primer sets DpActF1&R2 specific to D. phaseolorum derived from actine gene and PmCoxF1&R1 specific to P. manshurica derived from Cox region was successfully established for simultaneous detection of both the pathogens.
Triplex PCR for simultaneous detection of two fungal species namely B. maydis, D. maydis and insect Sitophilus zeamais associated with maize was performed with every possible combination of specific markers developed for each fungal pathogens and insect. Combination of specific markers BmItsF1&R2 derived from ITS for B. maydis, DmKbF3&R3 derived from kilbournase gene for S. maydis and SzCoF3&R3 derived from 12Smt DNA for S. zeamais were used for development of triplex PCR for simultaneous detection of two fungal species and one insect species associated with maize. The markers, BmItsF1&R2, DmKbF3&R3 and SzCoF3&R3 produced specific size of amplicon; 414bp, 135bp and 275bp, respectively for respective target fungal pathogens and insect.
All the imported transgenic lines were tested to ensure the absence of embryogenesis deactivator gene by PCR with primers specific to the cre-lox system. Plasmid cloned with cre sequence was used as positive control. In PCR amplification of cre sequence, the amplicon of 1031 bp size was amplified only in positive plasmid sample while, no amplicon of corresponding size was observed in any of these transgenic samples ensuring the absence of embryogenesis deactivator gene. The samples were also tested for specific transgenic elements using PCR/ Real-time PCR based GM diagnostics.
Staff
Scientist

Head of Division
Division of Plant Quarantine
Phone: 011-25802787
Email: celia.chalam(AT)icar.org.in, mailcelia(AT)gmail.com

Principal Scientist
Division of Plant Quarantine
Phone: 011-25802750
Email: Kavita.Gupta(AT)icar.org.in, kavita6864(AT)gmail.com

Principal Scientist
Division of Plant Quarantine
Phone: 011-25802752
Email: jameel.akhtar(AT)icar.org.in, jameelnbpgr(AT)gmail.com, jameelbau(AT)rediffmail.com

Principal Scientist
Division of Plant Quarantine
Phone: 011-25802751
Email: moolchand.singh-icar@nic.in, mchsingh@gmail.com

Senior Scientist
Division of Plant Quarantine
Phone: 011-25802731
Email: bharat.gawade-icar@nic.in

Senior Scientist
Division of Plant Quarantine
Phone: 011-25802724
Email: pardeep1-icar@nic.in

Senior Scientist
Division of Plant Quarantine
Phone: 011-25802273
Email: sangeetamicro@gmail.com

Scientist(SS)
Division of Plant Quarantine
Phone: 011-25802754
Email: pooja.kumari-icar@nic.in

Scientist(SS)
Division of Plant Quarantine
Phone: 011-25802725
Email: Bharat.Meena3-icar@nic.in, brrm1406@gmail.com
Technical

Chief Technical Officer
Division of Plant Quarantine
Phone: 011-25802727
Email: dharm.meena-icar@nic.in, dsmeenapqd@gmail.com

Assistant Chief Technical Officer
Division of Plant Quarantine
Phone: 011-25802749
Email: Smita.Jain-icar@nic.in



