DIVISION OF GERMPLASM CONSERVATION

Head of Division: Dr. Anju Mahendru Singh
Contact Numbers: 011-25802784 Ext. 784
Address: ICAR-National Bureau of Plant Genetic Resources, Pusa Campus, New Delhi – 110012, INDIA
E-mail:anju.singh@icar.org.in; nbpgr.conservation@gmail.com; head.dgc@gmail.com
Conservation of Plant Genetic Resources of India has been an activity as old as the Agriculture itself. However, organised conservation of plant genetic resources was started at the then Botany Division of the Indian Agricultural Research Institute (under the Indian Council of Agricultural Research) wayback in 1940s. With expansion of its activities, it was termed as the Plant Introduction section in 1946, Plant Introduction and Exploration Organization in 1956 and later as Plant Introduction Division in 1961 all headed by Dr Har Bhajan Singh. With further expansion of its activities and role in ICAR, the Plant Introduction Division was raised to the status of an independent national institute called the National Bureau of Plant Introduction in 1976 and then rechristened as the National Bureau of Plant Genetic Resources (NBPGR) in January, 1977. With enhanced PGR management responsibilities at the national level, the NBPGR distributed its activities among five distinct divisions viz., Plant Exploration and Collection, Germplasm Evaluation, Germplasm Conservation, Plant Quarantine and Genomic Resources with regular Heads of Divisions.
The Division of Germplasm Conservation (DGC) manages the National Genebank (NGB) of India which is responsible for the ex -situ conservation of germplasm collections in the form of seeds, vegetative propagules, tissue/cell cultures, embryos, gametes, etc.
Mandate
The Division of Germplasm Conservation is entrusted with the responsibility of conservation of Plant Genetic Resources for the posterity and sustainable use. The major objectives are:
- Maintain and operate different components of the National Genebank
- Function as the national repository for ex-situ conservation of indigenous and exotic Plant Genetic Resources (PGRs) in the form of orthodox and non-orthodox seeds, vegetative propagules, tissue/cell cultures, embryos, gametes etc.
- Issue a unique National Identity (Indigenous collection or IC number) to the indigenous germplasm deposited by different stakeholders
- Monitor conserved germplasm for viability, health and genetic stability
- Supply PGRs to the indenters
- Facilitate registration (soft protection) of PGRs with unique/novel/superior traits of economic/academic importance
- Conduct fundamental and applied research in ex-situ conservation of PGRs
- Develop human resources in ex-situ conservation and genebank management
Major Activities
Operation and Maintenance of National Genebank (NGB) Facility
The base collection in seed genebank is held in 12 long-term storage modules with a total capacity of upto 10,00,000 accessions. The cryogenebank includes seven cryo-tanks, each containing 1,000 litres of liquid nitrogen with a total capacity to store three lakh samples. These facilities are maintained round-the-clock by highly skilled technical staff of DGC.
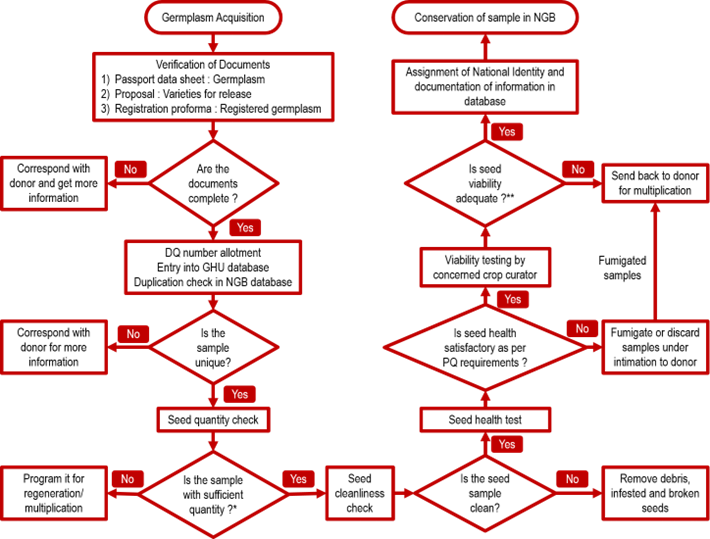
Germplasm Conservation in the Seed Genebank
Germplasm samples are acquired in the Germplasm Handling Unit through exploration and collection missions or from national and international research institutions and other stakeholders. The samples are processed for safe and pest free conservation. In the seed genebank, this is carried out by examination of seed quality, quantity, viability and seed health to ensure compliance with genebank standards for long-term conservation and achieved through seed cleaning, germination testing, drying and packing. A unique National Identity is given to the qualifying accession and the storage pouches are properly labelled along with passport details and location and finally stored in long-term-storage modules at -180C±20C at the designated place.


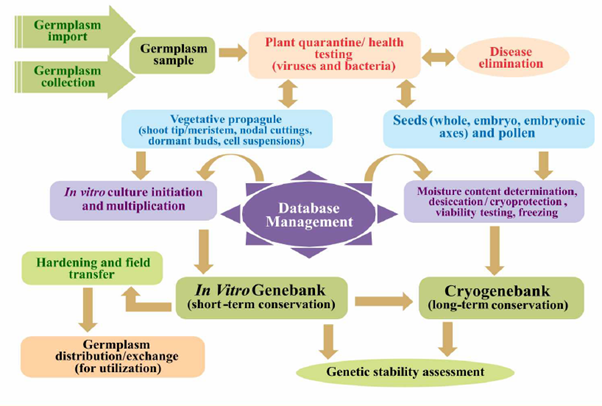
Germplasm Conservation in the in Vitro Active and Base Genebanks
The steps in the processing of the in-vitro conservation of difficult to conserve crops and species and clonally propagated germplasm to ensure pest-free conservation are depicted below:

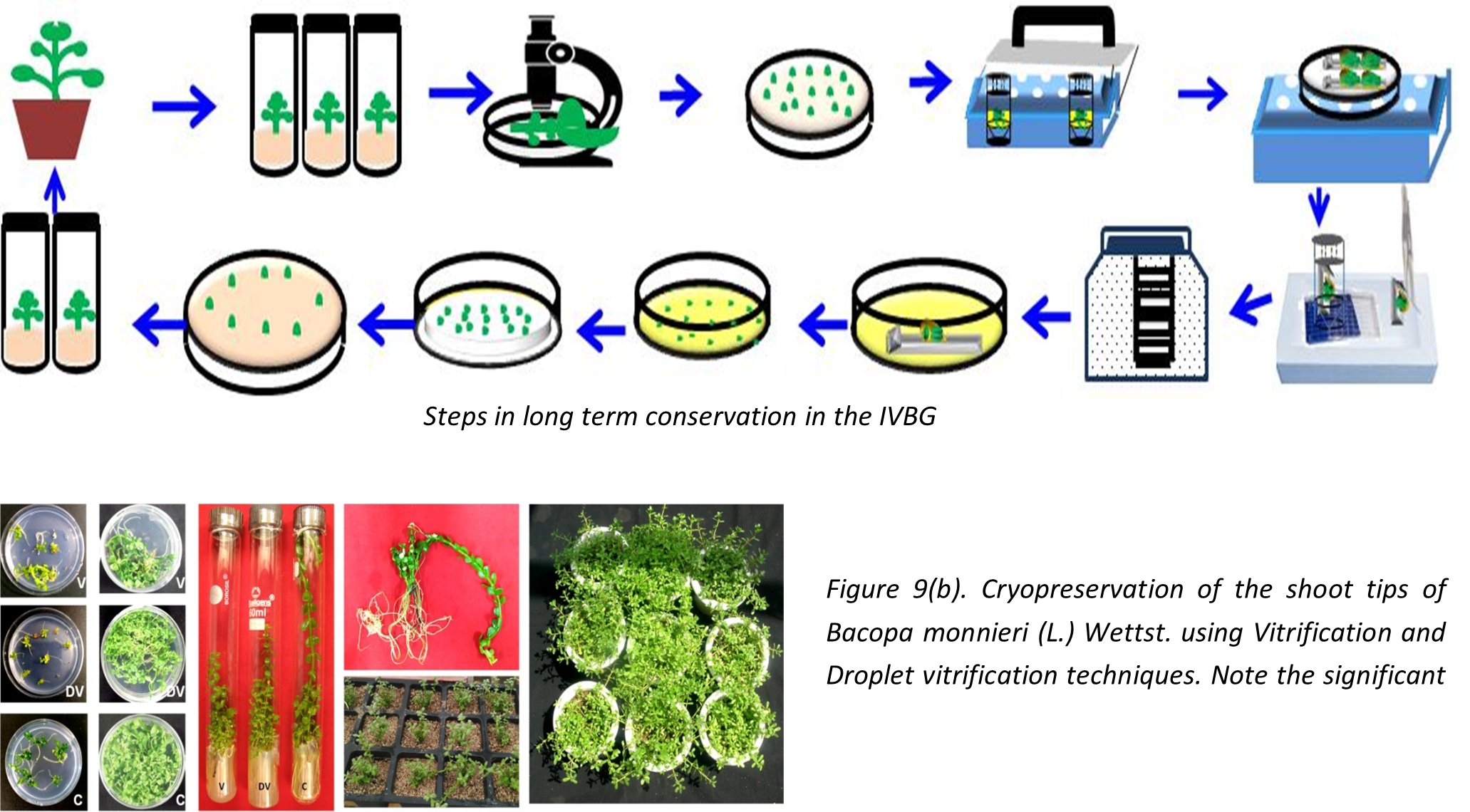
Germplasm Conservation in the Cryogenebank
While orthodox seeds can be easily conserved at -180C, the non-orthodox seed species present difficulty in conservation at this temperature. The non-orthodox seed species can be designated as highly recalcitrant (e.g. mango, litchi, jackfruit, mahua, jamun, mangosteen etc.), moderately recalcitrant (papaya, several citrus species, black pepper, banana etc.) and weakly recalcitrant (some citrus species, bael, custard apple etc.). Recalcitrant seeds at various developmental stages are reported to have varying degree of tolerance to desiccation. Cryopreservation of desiccation-sensitive tissues is only possible once the moisture content of explants is reduced to an optimal level with reasonably high viability and is able to survive freezing stresses as low as -196°C. Whole seeds, embryos, embryonic axes and pollen are preserved in the vapour phase of liquid nitrogen after careful assessment of desiccation tolerance and determining the critical moisture content of the tissue before cryopreservation in the Cryogenebank.
Data documentation and curation
Seed genebank management data such viability, moisture content, seed quantity, location in long-term storage module and date of storage are documented by the Genebank documentation unit in the Genebank Information Management System (GBIMS) developed in-house and maintained by AKMU. This data is curated by the different crop curators. The management data being linked to taxonomy and passport information databases in GBIMS enables curators to make informed decisions about monitoring and regeneration of conserved accessions as well as retrieve status of current holdings as reports. Similar data management system is also available for the in vitro genebank and cryogenebank.

Monitoring of conserved germplasm
For seed genebank, monitoring involves inspection of quality (seed viability) and quantity (number or weight) of germplasm accessions at perioding intervals during storage. The viability of conserved seeds decreases over time in storage and by monitoring accessions whose viability is falling below acceptable thresholds can be identified for regeneration. Similarly, retrieval of conserved seeds for research/utilization and germination testing results in depletion of seed quantity and can also be identified for regeneration. Similarly, in case of in vitro genebank for all cultures, genetic stability and health status is monitored at periodic intervals. For cryopreserved seeds and in vitro cryopreserved meristems too recurring viability tests are undertaken. All in vitro cultures screened periodically for identification and elimination of covert endogenous bacteria. Moreover, strategies such as meristem culture and cryotherapy are employed to retrieve virus free material.
Regeneration
Regeneration is the process of renewing conserved germplasm accessions by regenerating the plants from specific quantity of seeds or planting materials and re-collection of the harvested seeds or plant materials possessing the same genetic make-up as the original population. This is achieved in cooperation with Division of Germplasm Evaluation, NBPGR Regional stations, Research institutes including identified National Active Germplasm Sites. Regeneration helps in replenishing the accessions in the base collection and to increase the seed quantity in case the quantity of initial material is insufficient as per genebank standards. In case of in vitro conservation, regeneration of plantlets through rapid clonal multiplication is a key a prerequisite. Similarly, species specific optimized protocols for regeneration are key to practical conservation of diverse germplasm.
Distribution and supply
NGB facilitates the distribution of representative samples accessions according to indents received via Germplasm Exchange and Policy Unit (GEPU). The supply is dependent upon availability of the accession in sufficient seed quantity in seed genebank as well as absence of any policy restrictions. For germplasm preserved via in vitro conservation and cryopreservation, material is distributed as in vitro cultures. The material is distributed ensuring its arrival at the intended destination in excellent condition along with any associated information.

Plant Germplasm Registration
This involves registering, conserving, documenting and distributing promising trait-specific germplasm by giving due credit to developers and bringing them to public domain to enhance germplasm utilization. DGC facilitates registration such unique germplasm of academic, scientific and applied value from the inception of the programme in 1996 by processing the seed materials and the proposals. From 2018 this is done via a fully online system of filing registration applications, their scrutiny, review and communications at each stage called Germplasm Registration Information System (GRIS, http://www.nbpgr.ernet.in:8080/registration/).
Safety Duplicates and voucher samples
Apart from regular storage of accessions for long-term conservation, DGC also maintains samples to fulfil policy obligations and in accordance with MOUs with other agencies.- Conservation of safety duplicates of 10,235 accessions of lentil and pigeonpea from International Center for Agricultural Research in the Dry Areas (ICARDA).
- Repository for accessions of seed bearing trees species of forestry importance provided for Forest Research Institute (FRI).
- Storage of voucher samples of non-proprietary exotic germplasm arriving in India.
- Repository for voucher samples of cultivated plants and their wild relatives for National Biodiversity Authority (NBA).
Supportive research
The division also conducts fundamental research on several aspects of conservation science such as:- Studies on seed longevity, dormancy, germination, storage and processing.
- Development of new strategies and tools for efficient management of base collection in NGB.
- Development of protocols for (i) in vitro multiplication/micropropagation (ii) in vitro conservation (iii) cryopreservation using diverse explants (meristems, dormant buds, seeds, embryos/embryonic axis, pollen).
Human Resource Development
The division also engages in human resource development in the areas of seed conservation, seed science, in vitro conservation and cryopreservation through conduct of regular training programs, acting as resource personnel in external trainings and as a part of the PG School of ICAR-Indian Agricultural Research Institute.Public Outreach
The National Genebank being the crown jewel in the Indian National Plant Genetic Resources System (INPGRS), regularly hosts several dignitaries, policymakers and other stakeholders who are illuminated about its key role, and activities and the importance of supporting it.Salient Achievements
Germplasm assembly
Over the last four decades, NGB’s seed bank has grown into a colossal seed vault and has been catering to the crop improvement programmes of almost all agricultural and horticultural crops. Valuable germplasm of various crop species has been collected, acquired and assembled in NGB through an active collaboration with the partners in the national agricultural research system (NARS). As a result of all these efforts, the base collections in the NGB holds more than 4,60,000 accessions belonging to more than 1,800 species making it the second largest genebank in the world.
The seed genebank as of April 2024 holds 469380 accessions of 2150 different species. Similarly, the in vitro genebank is conserving 1,985 accessions as meristem cultures and the cryogenebank is holding 12,790 accessions as seeds, dormant buds and pollen.
Seed Genebank Status (as of April, 2024)
| Crop/Crop Group | No. of accessions | Crop/Crop Group | No. of accession | |
| Seed genebank | In vitro genebank | |||
| Cereals | 177205 | Tropical fruits | 449 | |
| Millets | 60435 | Temperate and minor tropical fruits | 390 | |
| Forages | 7522 | Tuber crops | 527 | |
| Pseudocereals | 8221 | Bulbous crops | 178 | |
| Legumes | 68881 | Medicinal & aromatic plants | 211 | |
| Oilseeds | 63508 | Spices and industrial crops | 230 | |
| Fibre | 17056 | Total | 1,985 | |
| Vegetables | 29504 | Cryobank | ||
| Fruits & Nuts | 302 | Recalcitrant and Intermediate | 7,784 | |
| M&AP | 9530 | Orthodox | 3,962 | |
| Ornamental | 743 | Dormant bud | 389 | |
| Spices, Condiments and Flavour | 3697 | Pollen | 655 | |
| Agroforestry | 1710 | Total | 12790 | |
| Duplicate safety Samples | 10235 | |||
| Trial Material (Wheat, Barley) | 10771 | |||
| Total | 469320 | |||
Monitoring of the viability of germplasm conserved in Seed genebank
| Crop | No. of acc. tested for viability | Initial viability range (%) | Present viability range (%) | No. of Acc. identified for regeneration | Crop | No. of acc. tested for viability | Initial iability range (%) | Present viability Range (%) | No. of Acc. identified for regeneration | |
| Rice | 1500 | 99 | 86 | 57 | Pearl millet | 678 | 82-100 | 80-98 | Nil | |
| Maize | 145 | 90-100 | 80-100 | 3 | Sorghum | 992 | 85-100 | 68-100 | 36 | |
| Barley | 213 | 80-100 | 70-100 | 4 | Fiber | 1120 | 65-100 | 60-100 | 254 | |
| Wheat | 533 | 94-100 | 80-100 | 6 | Forages | 589 | 20-100 | 10-100 | 120 | |
| Brinjal | 832 | 70-90 | 50- 80 | 21 | Medicinal & Aromatic plants | 143 | 20-100 | 20-100 | – | |
| Tomato | 484 | 70-90 | 70-90 | – | Pseudocereals | 619 | 85-100 | 85-100 | – | |
| chilli | 58 | 70-90 | 70-90 | – | Spices | 359 | 85-100 | 85-100 | – | |
| Okra | 109 | 70-90 | 70-90 | – | Ornamental | 113 | 30-100 | 30-100 | – | |
| Ridge gourd | 214 | 70-100 | 50-90 | 36 | Agroforestry | 01 | 20-100 | 20-100 | – | |
| Round gourd | 54 | 70-90 | 50-80 | 29 | Castor | 50 | 85-100 | 80-100 | – | |
| Cucumber | 453 | 70-100 | 70-100 | 65 | Groundnut | 224 | 80-100 | 80-100 | – | |
| Radish | 30 | 70-90 | 70-90 | – | Jatropha | 7 | 50-100 | 80-95 | – | |
| Pumpkin | 177 | 70-90 | 50-80 | 37 | Linseed | 157 | 88-100 | 80-100 | 2 | |
| Sponge gourd | 280 | 70-100 | 70-100 | – | Mustard | 250 | 95-100 | 90-100 | – | |
| Muskmelon | 100 | 70-100 | 70-100 | – | Niger | 351 | 80-100 | 80-100 | 6 | |
| Watermelon | 36 | 70-80 | 70-80 | – | Oilseed Brassica | 223 | 80-100 | 35-100 | – | |
| Ash gourd | 206 | 70-90 | 50-90 | 5 | Pongamia | 7 | 80-85 | 80-95 | – | |
| Lablab bean | 289 | 60-80 | 50-80 | 28 | Safflower | 97 | 60-100 | 60-100 | – | |
| Onion | 738 | 70-90 | 50-90 | 21 | Sesame | 1244 | 60-100 | 30-100 | 88 | |
| Cauliflower | 104 | 70-100 | 70-100 | – | Soybean | 193 | 60-100 | 50-100 | 10 | |
| KnolKhol | 08 | 70-100 | 70-100 | – | Sunflower | 7 | 80-100 | 85-100 | – | |
| Barnyard millet | 311 | 82-100 | 78-96 | – | Castor | 50 | 85-100 | 80-100 | – | |
| Little millet | 281 | 80-100 | 78-98 | – | Groundnut | 224 | 80-100 | 80-100 | – | |
| Proso millet | 103 | 88-100 | 84-100 | – | Lathyrus | 179 | 90-100 | 85-100 | – | |
| Horsegram | 145 | 86-100 | 80-100 | 02 | Mothbean | 138 | 90-100 | 85-100 | – | |
| Fababean | 184 | 86-100 | 85-100 | – | Ricbean | 341 | 90-100 | 85-100 | – | |
| Clusterbean | 464 | 90-100 | 80-100 | – | Grand total | 16,686 | 830 | |||
| Frenchbean | 279 | 90-100 | 85-100 | – |
Monitoring of the viability of germplasm conserved in Cryo Genebank
| 2019-20 | 2020-21 | 2021-22 | 2022-23 | 2023-24 | Total | |
| No of accessions monitored for viability | 49 (14) | 62 (10) | 45 (16) | 161 (37) | 86 (49) | 403 (126) |
| No. of accessions ascertained for pest- free status | 155 (42) | 134 (35) | 58 (19) | 201 (58) | 77 (33) | 625 (187) |

Germplasm Distribution during the last five years
| Crops (no. of accessions) | No. of acc. |
| Wild wheat (399) and Maize (150) | 549 |
| Triticale (1171) | 1171 |
| Wheat (2338) | 2338 |
| Chilli (3022), Solanum spp (278), Cucumis spp (42), Watermelon (35), tomato (151), musk melon (229), bitter gourd (277), cauliflower (285), Knol Khol (08), yard long bean (15), sponge gourd (38), bottle gourd (32), Luffa spp (1279), Snake Gourd (243), snap melon (53), round gourd (54), ash gourd (23), pumpkin (200), portulaca (10), radish (22), onion (01), beetroot (05) and broccoli (09) | 6311 |
| Faba Bean (121) and Pea (250) | 371 |
| Brassica (1195), Brassica (593), Brassica juncea (1000), Camelina sativa (10), Oilseed Brassica (2), Linseed (2705), Niger (3807), Pongamia (58), Safflower (7000), Sesame (5406), Sesame (1925), Soybean (7185) and Soybean (10) | 30,896 |
| Mungbean (241) | 241 |
| Chickpea (7165) | 7165 |
| Mungbean (20) | 20 |
| Mungbean (3968),Mothbean (70), Mothbean (1538), Frenchbean(132), Pigeonpea (180), | 5888 |
| Total | 54950 |
Plant Germplasm Registration
51 meetings of the Plant germplasm Registration ommittee chired by the DDG (crop Science), ICAR have been conducted by the Division with Head as the member-secretary. 2199 germplasm accessions having superior or novel traits belonging to 261 different species have been registered and given a unique INGR number.
| Crop group | Germplasm registered till (April 2019 to March 2024) | Present status till March 31st 2024 | No. of Species till March 31st 2023 |
| Cereals and Pesudocereals | 270 | 786 | 10 |
| Millets | 75 | 157 | 6 |
| Fibre and Forages | 16 | 130 | 17 |
| Grain Legumes | 82 | 221 | 24 |
| Vegetables | 59 | 139 | 26 |
| Oilseeds | 70 | 261 | 22 |
| Commercial Crops | 21 | 121 | 17 |
| M & AP & Spices and Masticatory | 46 | 136 | 59 |
| Fruits and Nuts | 33 | 75 | 24 |
| Tubers | 20 | 57 | 17 |
| Ornamentals | 43 | 99 | 30 |
| Narcotic/Beverages | 9 | 9 | 2 |
| Agro-forestry | 0 | 8 | 7 |
| Grand Total | 744 | 2199 | 261 |

Supportive Research
- In vitro cultures of Allium , Fragaria spp., Musa spp., Rubus spp., Ipomoea spp., indexed for viruses using serological and immunological techniques Direct Antigen coated-indirect-enzyme linked Immunobsorbent assay (DAC-ELISA, DAS-ELISA). PCR based techniques and electron microscopy.
- Meristem culture technique standardized for sweet potato and Allium for recovering virus-free plants.
- Cryotherapy as a means for obtaining virus-free material attempted in germplasm of banana, garlic and strawberry, with various degree of success.
- Periodic viability testing undertaken for cryopreserved seeds and in vitro cryopreserved meristems. Original viability retained in 5-31 years of cryostorage in species tested so far.
Improved protocols developed and/or standardized
Micropropagation/in vitro multiplication protocols
Micropropagation/in vitro multiplication protocols have been developed or refined using varied explants (shoot tips, nodal cuttings, floral meristems, seeds segments) and regeneration systems (axillary bud proliferation, organogenesis, somatic embryogenesis). Important crops and species include:- Fruits: Banana (Musa), almond (Prunus armenica), kiwi (Actinidia chinensis), mangosteen (Garcinia indica and G. cambogia), pear (Pyrus communis), mulberry (Morus spp.), Rubus spp.,woodapple (Aegle marmelos),
- Spices and Industrial crops: ginger Black pepper, betel vine etc. (Piper), cardamom (Elettaria cardamomum), ginger (Zingiber officinale), jojoba (Simmondia chinensis), turmeric (Curcuma spp.), vanilla (Vanilla planifolia)
- Tubers: Asian yams (Dioscorea esculenta, D. pentaphylla, D. hispida and wallichii), giant taro (Alocasia indica), Chinese potato (Coleus parviflorus), sweet potato (Ipomoea batatas), taro (Colocasia esculenta),
- Bulbs and Ornamentals: Chinese chives (Allium tuberosum, A. bakeri), Dahlia
- MAP and RET spp.: Bacopa monnieri, Coleus forskohlii, Curculigo orchoides, Gentiana kuroo, Kaempferia , Pogostemon patchouli, Rauvolfia canescens and Tylophora indica.
Protocols for in vitro Conservation
Various slow growth strategies such as low temperature incubation, use of osmotic agents, growth retardants, nutritional or hormonal manipulations etc. have been evaluated for different crop species.- Use of minimal media in Acorus calamus, Actinidia , Aegle marmelos, Allium spp., Aristolochia indica, Artocarpus lakoocha, Cholorophytum borivilianum, Centella asiatica, Curcuma spp., Colocasia esculenta Centella asiatica, Curcuma spp., Dioscorea spp., Ipomoea batatas, Kaempferia spp., Malus spp., Mentha spp., Morus spp., Piper spp., Plumbago spp., Pogostemon patchouli, Pyrus spp., Rubus spp., Tylophora indica,
- Low temperature incubation (4-20oC) in general found effective in prolonging subculture interval to 6-24 months in many species, especially those belonging to Allium, Fragaria, Gentiana, Musa, Picrorhiza, Rauvolfia, Swertia and Valeriana.
- Induction of storage organs in Alliums, ginger, turmeric, yams, taro, Kaempferia significantly increased storage period up to 20-24 months at 25oC. In vitro produced rhizomes, bulblets or micro- tubers could also be used for direct field planting with high success.
- Minor modifications of the media extended shelf life of cultures in Alocasia, Coleus Piper, Rauvolfia, Simmondsia chinensis, to 12-24 months at 25oC. In yams, small increase in cytokinin concentration helped extend survival of cultures beyond 14 months at 25o C. Mannitol at low levels prolonged subculture period to 16 months at 25oC in sweet potato, while mineral oil overlay in Bacopa monnieri cultures increased storage from 6 to 24 months.
- Protocols developed by using low-cost media worked effectively in banana, ginger, turmeric, galangal, without any loss of regeneration potential.
Protocols for Cryopreservation
- Cryopreservation protocols developed/refined using shoot tip/meristem explants derived from in vitro cultures in Allium hookeri, A. scorodoprasum, A. ramosum, A. chinense A. sativum, A tuberosum, Bacopa monnieri, Curcuma longa, Dioscorea bulbifera, D. deltoidea, Dahlia, Elettaria cardamomum, Gentiana kurroo, Malus, Musa, Prunus, Picrorhiza, Rauvolfia serpentina, Rubus and
- Studies on seed viability, moisture content, desiccation and freezing sensitivity conducted on cheura (Diploknema butyracea), jamun (Syzygium cuminii), phalsa (Grewia asiatica), wild apricot (Prunus armeniaca), Pilu (Salvadora oleoides), persica, Alangium salvifolium, Murraya paniculata, M. koeingii, Capparis decidua and grape fruit (Citrus paradisi), trifoliate orange (Poncirus trifoliata) and its hybrids, Citrus species, viz., Citrus sinensis, C. reticulata, C. aurantium, Citrumelo (Poncirus trifoliata × C. paradisi) and several other citrus spp and underutilized fruits. Seeds of Diploknema butyracea, Syzygium cuminii, Citrus jhambiri, Poncirus trifoliata were found to be recalcitrant while others showed intermediate storage behaviour.
- Basic cryobiological studies on morphological, physiological and biochemical parameters of difficult-to-store germplasm of intermediate and recalcitrant seed species (> 37 genera of 70 species) carried out in order to determine factors responsible for degree of recalcitrance.
- Base collection with sizeable indigenous variability established for Citrus (32 species), Buchanania lanzan, Capparis sp., Manilkara hexandra, Piper nigrum, Juglans regia, Prunus amygdalus, Jatropha curcas, Pongamia pinnata, Prunus armeniaca, Morus spp. and Malus cvs. (vegetative buds).
- Vast genetic wealth of Citrus existing in Northeast and Northwest India which includes rare, endangered, endemic species and important genotypes of other indigenous Citrus species have been cryopreserved by developing suitable cryopreservation protocols ensuring long-term safe conservation. Methods of desiccation-freezing, encapsulation and vitrification have been successfully applied in various Citrus species and cultivars. Comprehensive cryobase collection of more than 600 accessions of Indian Citrus germplasm has been established in cryogenebank that comprises rootstocks, wild, rare and endangered species such as indica, C. macroptera, C. megaloxycarpa, C. laptipes etc.
Identification keys
Identification keys for wild species of Swertia and Crotolaria conserved at NGB using seed characters viz.,color, size, shape, hilum position and spermoderm pattern were generated using scanning electron microscopy and light microscope.Seed deterioration studies
To understand the mechanism of seed deterioration a number of physiological and biochemical markers were studied in radish, Brassica spp., soybean, castor, Dalbergia, and Baliospermum montanum. The parameters studied include seed leachate conductivity, UV absorbancy, sugars, proteins, lipid peroxides and some reserve mobilizing as well as free-radical scavenging enzymes.Studies on seed storage behaviour
Studies on seed storage behaviour in Pongamia pinnata, Murraya paniculata, Saraca asoka, Swietinia macrophylla, Mimusops hexandra and Jatropha curcas were carried out. S. macrophylla and M. hexandra seeds showed intermediate behaviour while S.asoka showed recalcitrant behaviour and P. pinnata, M. paniculata and J. curcas had orthodox behaviour.Software tools
 |
Software packages ‘viabilitymetrics’ and ‘geriminationmetrics’ were developed in the popular free and open source statistical computing language `R` to aid computation of germination indices, viability statistics and curve fitting of cumulative germination and seed survival curves.
Similarly, a `R` package `PGRdup has also been developed for identification of probable/possible duplicates in Plant Genetic Resources (PGR) collections using passport data. Other `R` packages developed includes `augmentedRCBD` for analysis of data generated from experiments in augmented randomised complete block designs and `EvaluateCore` for quality evaluation of core collection.Testing Genetic Stability of In Vitro Conserved and Cryopreserved Germplasm
- Plants re-established in soil following various period of in vitro conservation/ cryopreservation were found to maintain their fruit characters (Musa), special chemical constituents (Coleus, Pogostemon, Bacopa monnieri), morphological characters (sweet potato, garlic, galangal) and genomic integrity (Bacopa, banana, ginger, turmeric, cardamom, yams, taro, etc.).
- Thus, conservation protocols developed at TCCU have been found to maintain genetic integrity which is of utmost importance in germplasm conservation programme anywhere in the world.
Consultancy for establishment of Medium term Storage Modules
Consultancy regarding the technical know-how of low cost conservation, Inputs and detailed layout and drawing, for specifications of MTS establishment is given by Division of Germplasm Conservation to interested parties. Following organizations were given the consultancy during 2019-2024:- Directorate of Research, SKUAST, Jammu, J&K
- Basmati Export Development Foundation, Meerut, Uttar Pradesh
- ICAR-IIHR, Bengaluru, Karnataka
- UAS, Dharwad, Karnataka
- AAU, Jorhat, Assam
- Keylang, Lahaul and Spiti, Himachal for Rail India Economic &Technical services, Gurgaon
- ICAR-NBPGR RS, Thrissur, Kerala
- ICAR-IIMR, Hydrabad, Telangana
- ICAR-NRCSS, Tabiji, Ajmer
- Forest Department, Arunachal Pradesh
- National Institute Abiotic Research, Raipur
- National Institute of Plant Genome Research, New Delhi
- Chief Conservator of Forest, Meghalaya
- Joint Director of State Agriculture Research Station, Nagaland
- KVK Zunheboto, Nagaland University, Lumami
- Bharti Vidhypeeth University, Pune, Maharashtra
- CSDS, Tata Institute of Social Sciences, Mumbai
- Department of Agricultural Biotechnology, AAU, Jorhat, Assam
- Supply spares parts for MTS module, ICAR-NBPGR RS, Bhowali, Uttrakhand
- Technical know-how for MTS and technical specifications of dehumidifier of MTS module was provided to ICAR-IGFRI, Jhansi, Uttar Pradesh
- Members in Committee for establishment of Genebank at PPV&FR Authority Bhawan

Facilitation of student visits
| Different institutes/ SAUs/Private/others | No. of Institutes | No. of Students visited |
| CAUs | 05 | 206 |
| ICAR | 01 | 25 |
| Universities (SAU/ Traditional /Private) | 246 | 10101 |
| Others (Trainees/Awardee farmers, Forest rangers etc.) | 07 | 362 |
| Grand Total | 159 | 10694 |

Awards/Honours
Scientist Name | Year | Award name | Conferring agency |
Dr. Sandhya Gupta | 2023 | Fellow of the Linnaean Society of London | Linnaean Society of London |
Dr. S K Malik | 2020 | Fellowship of the Indian Society of Plant Genetic Resources | Indian Society of Plant Genetic Resources New Delhi |
Dr. S K Malik | 2023 | Fellowship of the Indian Society of Citricultue (Asian Citrus Congress) | Indian Society of Citricultue, Nagpur |
Dr. Anjali Kak Koul | 2021 | Fellow, Indian Society of Plant Genetic Resources | Indian Society of Plant Genetic Resources |
Dr. Sherry R Jacob | 2023 | Fellow, Indian Society of Plant Genetic Resources | Indian Society of Plant Genetic Resources |
J Arvind | 2020 | Netaji Subhas-ICAR International Fellowship | ICAR |
Dr. Vartika Srivastava | 2020 | Young Woman Scientist Award 2020 | Agro Environmental Development Society June 2020 |
Dr. Era Malhotra | 2020 | Young Scientist Award | Society for Scientific Development in Agriculture and Technology |
Dr. Era Malhotra | 2024 | Young Scientist Award | Agro Environmental Developmental Society |
Dr. Subhash Chander | 2021 | Young scientist award | Agro Environmental Development Society |
Dr. Gowthami R | 2024 | Dr R S Paroda Young Scientist Award | ISPGR |
Dr. Padmavati G Gore | 2020 | Recipient of Young Scientist Travel grant award for participating in Third Jack R Harlan International Symposium in Montpellier, France (2019) | Agropolis Foundation |
Dr. Padmavati G Gore | 2021 | Research wizard award at Institute of Life Sciences, Bhubaneswar, Odisha, 2021. | Agrivision |
Padmavati G Gore | 2024 | Dr R S Paroda Young Scientist Award | ISPGR |
Publications/Research Papers
2024
- Nair B, V Biradar, V Nagaich, CM Singh, BK Singh, SB Kumre, KM Shah, ND Tekale, RA Jadhav, A Tripathi, S Kumar, J Aravind, K Gupta, A Kumar and V Kaur (2024) Multi-environment screening of Linum germplasm collection for dissecting the potential of bud fly (Dasyneura lini Barnes) resistance and assembling a reference set for efficient utilization in genetic improvement. Ind. Crops Prod. 207: 117743. https://doi.org/10.1016/j.indcrop.2023.117743. (NAAS Rating-11.9)
- Bansal S, MK Sharma, P Joshi, EV Malhotra, M Latha & SK Malik (2024) An efficient direct organogenesis protocol for in vitro clonal propagation of Rubia cordifolia L. Industrial Crops & Products 208: 117856. (NAAS Rating-11.9)
- Malhotra EV, S Sharma, SC Mali & S BAnsal (2024) A A Droplet vitrification Cryopreservation protocol for conservation of hops (Humulus lupulus) genetic resources. Cryobiology (accepted March 2024) (NAAS Rating-8.9)
- Rane P , M Thakre, KM Verma, C Kumar, J Prakash, V Srivastava, PR Shashank, N Murukan, G Chawla, PK Mandal, H Kumar, AK Jadhav, E Varghese, VB Patel & SK Singh (2024) Studies on pollen micro-morphology, pollen storage methods, and cross-compatibility among grape (Vitis spp.) genotypes. Frontiers in Plant Science, 15, https://doi.org/10.3389/fpls.2024.1353808 (NAAS Rating-11.6)
- Srivastava V, B Panis & A Agrawal (2024) Droplet vitrification: A lifeline for long-term conservation of threatened species Garcinia indica. In vitro Cellular and Developmental Biology Plant (Accepted March, 2024) (NAAS Rating-8.6)
- Singh Sumit K, S Singhal, J P Jaiswal, U Basu, A N Sahi and Anju M Singh*.(2024). Physico-Chemical and Rheological Trait-Based Identification of Indian Wheat Varieties Suitable for Different End-Uses. Foods, 13(7):1125-.
2023
- M Sahu, N. Belsariya A Kak and Sujit (2023) Variability pattern and its distribution among germplasm accession of oat (Avena sp. L.). Forage Research 49(1): pp. 54-63. https://forageresearch.in/wp-content/uploads/2023/09/54-63-MAYURI-SAHU.pdf (NAAS Rating-4.76)
- Sharma L, Alam N. M, Roy, S, Satya P, Kar G, Ghosh, S, Goswami T and Majumder, B (2023). Optimization of alkali pretreatment and enzymatic saccharification of jute (Corchorus olitorius L.) biomass using response surface methodology. Bioresource Technology, 386,p128318 (NAAS Rating-17.4)
- Kaur V, SS Gomashe, J Aravind, SK Yadav, Sheela, D Singh, SS Chauhan, V Kumar, B Jat, NR Tayade, A Saroha, N Kaushik, S Langyan, M Singh, DP Wankhede, K Singh, A Kumar and GP Singh (2023) Multi-environment phenotyping of linseed (Linum usitatissimum L.) germplasm for morphological and seed quality traits to assemble a core collection. Ind. Crops Prod. 206: 117657. https://doi.org/10.1016/j.indcrop.2023.117657. (NAAS Rating-11.9)
- Sonia, V Kaur, SK Yadav, SS Arya, J Aravind, SR Jacob and RK Gautam (2023) Development and evaluation of barley mini-core collection for salinity tolerance and identification of novel haplotypic variants for HvRAF. Plant Soil, November. https://doi.org/10.1007/s11104-023-06397-6. (NAAS Rating-10.9)
- Saroha A, SS Gomashe, V Kaur, D Pal, S Ujjainwal, J Aravind, M Singh, S Rajkumar, K Singh, A Kumar and DP Wankhede (2023) Genetic dissection of thousand-seed weight in linseed (Linum usitatissimum L.) using multi-locus genome-wide association study. Front. Plant Sci. 14: 1166728. https://doi.org/10.3389/fpls.2023.1166728. (NAAS Rating-11.6)
- Kaur V, M Singh, DP Wankhede, K Gupta, S Langyan, J Aravind, B Thangavel, SK Yadav, S Kalia, K Singh and A Kumar (2023) Diversity of Linum genetic resources in global genebanks: From agro-morphological characterisation to novel genomic technologies – A review. Front. Nutr. 10: 1165580. https://doi.org/10.3389/fnut.2023.1165580. (NAAS Rating-11)
- Kumar S, S Basu, J Aravind and A Anand (2023) Assessment of storage potential of onion varieties using variables extracted from a mathematical model 4-parameter hill function (4-PHF). Seeds 2: 195–207. https://doi.org/10.3390/seeds2020015.
- Bansal S, MK Sharma, P Joshi, EV Malhotra & SK Malik (2023) Meta-topolin mediated enhanced in vitro propagation and genetic integrity assessment in sweet potato (Ipomoea batatas (L.) Lam). South African Journal of Botany, 157: 27-36. (NAAS Rating-9.1)
- Bansal S, MK Sharma, S Singh, P Joshi, P Pathania, EV Malhotra, Rajkumar S and P Misra (2023) Histological and molecular insights in to in vitro regeneration pattern of Xanthosoma sagittifolium. Scientific Reports, 13: 5806. (NAAS Rating-10.6)
- Chander S, D Ghosh, VC Tyagi, CR Chethan, D Pawar, Y Gharde … & PK Singh (2023). Imazethapyr-Resistant Jungle Rice (Echinochloa colona) in Soybean Growing Belt of Central India: A Case Study. Agricultural Research, 12(3):298–307. (NAAS Rating-7.4)
- Chander S, R Gowthami, R Pandey, M Shankar & A Agrawal (2023) Cryoconservation of in vitro grown shoot tips of Cicer microphyllum: A crop wild relative of chickpea. CryoLetters, 44(6): 360-368. (NAAS Rating-7)
- Choudhary R, SK Malik, R Chaudhary & AA Rao (2023) Optimized recovery of cryostored dormant buds of mulberry germplasm. Plants, 12: 225-238. (NAAS Rating-10.5)
- Gowthami R, N Sharma, R Chandra, JS Kurian, EV Malhotra & A Agrawal (2023) Development of an improved and simple shoot tip cryoconservation protocol for cryobanking of Bacopa monnieri (L.) Wettst. germplasm. In Vitro Cellular Developmental Biology-Plant, 59: 744–756. (NAAS Rating-8.6)
- Gowthami R, S Chander, R Pandey, M Shankar & A Agrawal (2023). Development of efficient and sustainable droplet-vitrification cryoconservation protocol for shoot tips for long-term conservation of Dahlia germplasm. Scientia Horticulturae, 321, 112329. https://doi.org/10.1016/j.scienta.2023.112329 (NAAS Rating-10.3)
- Gowthami R, S Chander, R Pandey, M Shankar & A Agrawal (2023) Development of cryoconservation protocol for in vitro shoot bases of Allium ampeloprasum L. for long-term conservation. Indian Journal of Plant Genetic Resources. 36(3), 396-401. (NAAS Rating-5.17)
- Nagar A, Gowthami R, Sureja AK, Munshi AD, Verma M, Singh AK, Mallick N, Singh J, Chander S, Shankar M, Pathania P and Rajkumar S (2023) Simple cryopreservation protocol for Luffa pollen: enhancing breeding efficiency. FrontIiers in Plant Sciences, 14:1268726. doi: 10.3389/fpls.2023.1268726 (NAAS Rating-11.6)
- Panda RR, A Pandey, S Gupta & KC Bhatt (2023) Use of fruit and seed characters for identification of edible Artocarpus species in India. Indian Journal of Plant Genetic Resources, 36(02): 309-312. (NAAS Rating-5.17)
- Puneeth GM, Gowthami R, R Vasudeva, KM Laxmisha & S Archak (2023) Circa situm Conservation of Tree Genetic Resources: A Case Study from the Central Western Ghats of Karnataka. India. Indian Journal of Plant Genetic Resources, 36(03):415-21. (NAAS Rating-5.42)
- Rane P, M Thakre, MK Verma, J Prakash, C Kumar, V Srivastava, PR Shashank & N Murukan N (2023). Standardization of pollen collection method and in vitro pollen germination media in grapes. The Pharma Innovation Journal, 12(12): 3159-3161 (NAAS Rating-5.23)
- Sagore B, K Singh, J Prakash, V Srivastava, M Vignesh & BK Yadav (2023) Elucidating the effect of plant bioregulators on embryo maturation for shortening the breeding cycle in papaya. Indian Journal of Horticulture, 80(3): 233-238. DOI : 10.58993/ijh/2023.80.3.1 (NAAS Rating-6)
- Sagore B, K Singh, J Prakash, SK Singh, V Srivastava, M Vignesh & BK Yadav (2023). Effect of plant growth regulators on fruit quality of papaya. The Pharma Innovation Journal, 12(8): 481-485 (NAAS Rating-5.23)
- Shankar M, R Gowthami, K Tripathi, DA Deepak, S Barpete & A Agrawal (2023) In vitro germination and cryopreservation technique for long-term pollen conservation of underutilized legume: Grasspea (Lathyrus sativus). Indian Journal Agricultural Sciences, 93 (2): 205–209. (NAAS Rating-6.4)
- Sreekanth D, D Pawar, R Kumar, P Ratnakumar, S Sondhia, PK Singh, JS Mishra, S Chander, N Mukkamula & BK Kumar (2023). Biochemical and physiological responses of rice as influenced by Alternanthera paronychioides and Echinochloa colona under drought stress. Plant Growth Regulation, 1-19. https://doi.org/10.1007/s10725-023-01089-8 (NAAS Rating-10.2)
- Vanniarajan C, P Magudeeswari, R Gowthami, SM Indhu, KR Ramya, K Monisha, MA Pillai, N Verma & Yasin JK (2023) Assessment of genetic variability and traits association in pigeonpea [Cajanus cajan (L.) Millsp.] germplasm. Legume Research, 46(10): 1280-1287. (NAAS Rating-6)
- Harisha R, Sumit K Singh, A K Ahlawat AK, S Narwal, J P Jaiswal, J B Singh,…., A Mahendru-Singh*. (2023). Elucidating the effects on polyphenol oxidase activity and allelic variation of polyphenol oxidase genes on dough and whole wheat-derived product color parameters. International Journal of Food Properties, 26(2):2716–31.
- Rai A, Santosh K Singh, Sumit K Singh, Poornima Sharma, Arvind K Ahlawat, Sung-Soo-Haan and Anju Mahendru Singh*. (2023). Analysis of genetic diversity and population structure using glutenin protein markers in various wheat varieties. Plant Genetic Resources, 21(1): 28 – 36, DOI: https://doi.org/10.1017/S1479262123000382.
2022
- Thribhuvan, R., S. P. Singh, M S. Sankar, Anju M Singh, M. Mallik, T Singhal, J Kumar Meena and C. T. Satyavathi. (2022). Combining ability and heterosis studies for grain iron and zinc concentrations in pearl millet [Cenchrus americanus (L). Morrone]. Frontiers in Plant Science; DOI 10.3389/fpls.2022.1029436.
- Rathan, N D, Hari Krishna, R K Ellur, D Sehgal, V Govindan, J P Jaiswal, J B Singh, S V S Prasad, D Ambati, S K Singh, K Bajpai and A Mahendru-Singh*. (2022). Genome wise association study identifies loci and candidate genes for grain micronutrients and quality traits in wheat (Triticum aestivum ). Scientific Reports, 12: 7037 http://doi.org/10.1038/s41598-022-10618-w.
- Kumar,S, S R Jacob, R R Mir, V K Vikas, P Kulwal, T Chandra, S Kaur, U Kumar, S Kumar, S Sharma, R Singh, S Prasad, A Mahendru-Singh, A K Singh, J Kumari, M S Saharan, S C Bhardwaj, M Prasad, S Kalia and K Singh (2022). Indian wheat genomics initiative for harnessing the potential of wheat germplasm resources for breeding disease resistant, nutrient dense and climate- resilient cultivars. Frontiers in Genetics; http:// doi/10.3389/fgene.2022.834366.
- Kaushik, M., E, Mulani, A Mahendru-Singh, G Makharia, S Mohan and P K Mandal (2022). Comparative Expression Profile of Genes Encoding Intolerant Proteins in Bread vs. Durum Wheat During Grain Development. J Plant Growth Regul (2022). https://doi.org/10.1007/s00344-022-10785-0.
- Awasthi,T, N Singh, AS Virdi, A Mahendru-Singh and AK Ahlawat (2022). Effect of solvents and supercritical‐CO2 extraction of lipids on physico‐chemical, functional, pasting and rheological properties of hard, medium hard and soft wheat varieties. International Journal of Food Science & Technology, 57(8):5057-5067
- Kumar, T P J, A K Ahlawat, S K. Singh, N. D. Rathan, N Bedi and A Mahendru-Singh* (2022). Quality evaluation of near isogenic lines of wheat developed through marker assisted backcross breeding for grain softness. Indian J. Genet. Plant Breed., 82(1): 56-64; doi/10.31742/ IJGPB.82 .1.8.
- Chandra, S, S P Singh, C T Satyavathi, S M Sankar, Anju Mahendru-Singh and J S Bhat. (2022). Genetics of fertility restoration for the A1 cytoplasmic genic male sterility system in pearl millet (Pennisetum glaucum (L.) R. Br.). Indian J. Genet. Plant Breed., 82(1): 65-72. doi: 10.31742 IJG PB.82.1.9.
- Arya L, RK Narayanan, A Kak, CD Pandey, M Verma and V Gupta (2022) ISSR marker based genetic diversity in Morinda spp. for its enhanced collection, conservation and utilization. Genet Resour Crop Evol 69: 1585–1593. Doi.org/10.1007/s10722-021-01321-2 (NAAS Rating-8)
- Toppo R, M Sahu, A Kak and N. Belsariya (2022) Assessment of genetic diversity among oat (Avena sativa L.) Germplasm accessions. Forage Research 48(1): pp. 62-68. https://forageresearch.in/wp-content/uploads/2022/06/62-68.pdf (NAAS Rating-4.76)
- Yadav R, Partha Ray Choudhury, Anjali Kak Koul, Veena Gupta, Ashok Kumar and Devendra K Yadava (2022). Status of Genetic Resources in Oilseed Crops and Their Potential Use for Making India Atmanirbhar in Edible Oils. Indian J. Plant Genet. Resour. 35(3): 85–89 (2022) https://doi.org/10.5958/09 (NAAS Rating-5.54)
- Yadav R, Partha Ray Choudhury, Anjali Kak Koul, Veena Gupta, Ashok Kumar and Devendra K Yadava (2022). Status of Genetic Resources in Oilseed Crops and Their Potential Use for Making India Atmanirbhar in Edible Oils. Indian J. Plant Genet. Resour. 35(3): 85–89 (2022) https://doi.org/10.5958/09 (NAAS Rating-5.54)
- Venugopalan V. K, Nath R, Sengupta K, Pal A. K, Banerjee S, Banerjee P, Chandran M. A. S, Roy S, Sharma L, Hossain A and Siddique K. H. M (2022). Foliar spray of micronutrients alleviates heat and moisture stress in lentil (Lens culinaris Medik) grown under rainfed field conditions. Frontiers in plant science, 13, p.847743. (NAAS Rating-11.6)
- Barman D, Chakraborty A, Das P. K, Roy S, Saha R, Mazumder S.P, Bandyopadhyay S, Singh A. K, Mitra S, Kundu D.K, Bagui A, Murthy C.S, Rao P.V.N, Choudhury, S and KarG (2022). Net ecosystem CO2 exchange from jute crop (Corchorus olitorius L.) and its environmental drivers in tropical Indo Gangetic plain using open path eddy covariance Technique. Environmental Monitoring and Assessment, 194:251. (NAAS Rating-9)
- Thakur M, Parveen S, Divte P.R, Mitra R, Kumar M, Gupta C. K, Kalidindi U, Bansal R, Roy S, Anand A, Singh B (2022). Metal tolerance in plants: Molecular and physicochemical interface determines the “not so heavy effect” of heavy metals. Chemosphere, 287: 131957. (NAAS Rating-14.8)
- Nanjundan J, J Aravind, J Radhamani, KH Singh, A Kumar, AK Thakur, K Singh, KN Meena, RK Tyagi and D Singh (2022) Development of Indian mustard [Brassica juncea (L.) Czern.] core collection based on agro-morphological traits. Genet. Resour. Crop Evol. 69: 145–62. https://doi.org/10.1007/s10722-021-01211-7. (NAAS Rating-8)
- Saroha A, D Pal, V Kaur, S Kumar, A Bartwal, J Aravind, J Radhamani, S Rajkumar, R Kumar, SS Gomashe, A Sengupta and DP Wankhede (2022) Agro-morphological variability and genetic diversity in linseed (Linum usitatissimum L.) germplasm accessions with emphasis on flowering and maturity time. Genet. Resour. Crop Evol. 69: 315–33. https://doi.org/10.1007/s10722-021-01231-3. (NAAS Rating-8)
- Kaur V, J Aravind, Manju, SR Jacob, J Kumari, BS Panwar, N Pal, JC Rana, A Pandey and A Kumar (2022) Phenotypic characterization, genetic diversity assessment in 6,778 accessions of Barley (Hordeum vulgare L. ssp. vulgare) germplasm conserved in national genebank of India and development of a core set. Front. Plant Sci. 13: 771920. https://doi.org/10.3389/fpls.2022.771920. (NAAS Rating-11.6)
- Langyan S, R Bhardwaj, J Radhamani, R Yadav, RK Gautam, S Kalia and A Kumar (2022) A quick analysis method for protein quantification in oilseed crops: A comparison with standard protocol. Front. Nutr. 9: 892695. https://doi.org/10.3389/fnut.2022.892695. (NAAS Rating-11)
- Saroha A, D Pal, SS Gomashe, Akash, V Kaur, S Ujjainwal, S Rajkumar, J Aravind, J Radhamani, R Kumar, D Chand, A Sengupta and DP Wankhede (2022) Identification of QTNs associated with flowering time, maturity, and plant height traits in Linum usitatissimum l. using genome-wide association study. Front. Genet. 13: 811924. https://doi.org/10.3389/fgene.2022.811924. (NAAS Rating-9.7)
- Agrawal A, N Sharma, S Gupta, S Bansal, V Srivastava, EV Malhotra, S Chander, R Gowthami & K Singh (2022) Biotechnological applications for plant germplasm conservation at ICAR-National Bureau of Plant Genetic Resources, India – Recent achievements. Acta Horticulturae, 1339: 29-41.
- Agrawal A, R Gowthami R, S Chander & V Srivastava (2022) Sustainability of In vitro genebanks and cryogenebanks. Indian Journal of Plant Genetic Resources, 35(3): 180-184 (NAAS Rating-5.7)
- Bansal S, A Kumar, AA Lone, MH Khan, EV Malhotra & R Singh (2022) Development of novel genome wide simple sequence repeats (SSR) markers in Bunium persicum. Industrial crops and products, 178: 114625 (NAAS Rating-11.9)
- Chethan CR, RP Dubey, S Chander, D Pawar D, D Ghosh & PK Singh (2022) Harnessing the full potential of low-dose high-potency (LDHP) herbicide molecules by standardized spraying technique in rice and wheat. Indian Journal of Weed Science, 54(2): 146-150. (NAAS Rating-5.42)
- Chethan CR, VK Tewari, AK Shrivastava, B Nare, SP Kumar, RP Dubey, PK Singh, D Ghosh, S Chander & S Dasari (2022) Optimization of Potato Sprout Orientation Angle and Effective Weed Management Practice to Produce Higher Economical Tuber Yield from Cut Tuber Planting. Potato Research, 66:195-213. (NAAS Rating-8.9)
- Deepak DA, EV Malhotra, M Shankar, A Agrawal (2022). Improved Micropropagation Protocol and Molecular Marker Based Genetic Stability Assessment of Black Pepper (Piper nigrum L.). Indian Journal of Plant Genetic Resources, 35: 264-274 (NAAS Rating-5.17)
- DN Yadav, S Tushir, S Sethi, NA Mir, R Wadhwa, S Bansal (2022) A superior approach for production of protein isolates from de-oiled soy meal and its comparison with conventional method. International Journal of Food Science and Technology, 57: 6245-6254 (NAAS Rating-9.3)
- Gupta S & Biraji VK (2022) In vitro propagation of Artocarpus lakoocha Roxb.: effect of meta-topolin on shoot parameters. Acta Horticulturae, 1339. DOI 10.17660/ActaHortic.2022.1339.2
- Malhotra EV, R Jain, S Tyagi, K Venkat Raman, S Bansal, D Pattanayak (2022). Identification of dynamic microRNA associated with systemic defence against Helicoverpa armigera infestation in Cajanus scarabaeoides, Pest Management Science, 78: 3144-3154 (NAAS Rating-10.1)
- Malhotra EV, R Jain, S Tyagi, K Venkat Raman, S Bansal, R Aminedi, D Pattanayak (2022). Comparative analysis of herbivory responsive miRNAs to delineate pod borer (Helicoverpa armigera) resistance mechanisms in Cajanus cajan and its wild relative Cajanus scarabaeoides, Plant Cell Reports, 41: 1147–1161 (NAAS Rating-12.2)
- Pandey A, PK Malav, DP Semwal, S Chander, R Gowthami & KM Rai (2022) Repository of Allium Genetic Resources at ICAR-NBPGR: Prospects and Challenges for Collection and Conservation. Indian Journal of Plant Genetic Resources, 35(3): 185-190. (NAAS Rating-5.17)
- Parimalan R, DP Wankhede, R Subramani, V Chinnusamy, SK Malik, MJ Baig, K Singh & R Henry (2022) Evolution of an intermediate C4 photosynthesis in the non foliar tissues of the Poaceae. Photosynthesis Research, https://doi.org/10.1007/s11120-022-00926-7. (NAAS Rating-9.7)
- Pawar D, S Dasari, S Chander S, CR Chethan, S Sondhia & PK Singh (2022) Effect of weed interference on rice yield under elevated CO2 and temperature. Indian Journal of Weed Science, 54(2): 129–136. (NAAS Rating-5.42)
- Rana JC, SK Malik & DE Falcis (2022) Building value chains for enhanced PGR utilization and sustainable food systems. Indian Journal of Plant Genetic Resources, 35: 100-106. (NAAS Rating-5.54)
- Ranjan P, P Brahmi, V Tyagi, JK Ranjan, V Srivastava, SK Yadav, SP Singh, S Singh, PC Binda, SK Singh & K Singh (2022) Global interdependence for fruit genetic resources: status and challenges in India. Food Security, 14: 591–619. https://doi.org/10.1007/s12571-021-01249-6 (NAAS Rating-12.7)
- Sharma N, EV Malhotra, R Chandra, R Gowthami, SM Sultan, S Bansal, M Shankar & A Agrawal (2022). Cryopreservation and genetic stability assessment of regenerants of the critically endangered medicinal plant Dioscorea deltoidea Wall. ex Griseb. for cryobanking of germplasm. In Vitro Cellular & Developmental Biology – Plant, 58: 521–529 (NAAS Rating-8.6)
- Sreekanth D, D Pawar, CR Chethan, PK Singh, S Sondhia, S Chander & MC Singh (2022). Indian quarantine weeds invasiveness assessment using bio-security tool: Weed Risk Assessment. Indian Journal of Weed Science, 54(2): 110–115. (NAAS Rating-5.42)
- Srivastava V, KC Bhatt KC & A Agrawal (2022) In vitro medium-term conservation of Garcinia indica: a tropical recalcitrant seeded fruit tree of India. In Vitro Cellular Deelopmental Biology-Plant, 58: 876–887. https://doi.org/10.1007/s11627-022-10288-3 (NAAS Rating-8.6)
- Srivastava V, S Hajong, R Chandora & A Agrawal (2022) Desiccation and freezing tolerance of recalcitrant seeds and embryonic axes of Prunus napaulensis (Ser.) Steud.: a crop wild relative of cherry. Genetic Resources & Crop Evolution, 69: 1571–1583. https://doi.org/10.1007/s10722-021-01320-3 (NAAS Rating-8)
- Yeddula N, SE Topno, V Srivastava, V Bahadur, VM Prasad & SK Singh (2022) Effect of chemical priming on seed germination and seedling growth in papaya (Carica papaya L.). Pharma Innovation, 11(5):2542-2546. (NAAS Rating-5.23)
2021
- Satya P, Sarkar D, Vijayan J, Ray S, Ray D. P, Mandal N. A, Roy S, Sharma, L, Bera A, Kar C. S, Mitra J and Singh N. K (2021). Pectin biosynthesis pathways are adapted to higher rhamnogalacturonan formation in lignocellulosic jute (Corchorus spp.) Plant Growth Regulation https://doi.org/10.1007/s10725-020-006736 (NAAS Rating-10.8)
- Rathan, N D, D Sehgal, K Thiyagarajan, R Singh, A Mahendru-Singh* and V Govindan (2021). Identification of Genetic Loci and Candidate Genes Related to Grain Zinc and Iron Concentration Using a Zinc-Enriched Wheat ‘Zinc-Shakti’. Frontiers in Genetics, https://doi.org/10.3389/fgene.2021.652653.
- Krishnappa, G., N D Rathan, D Sehgal, A K Ahlawat, S K Singh, R B Shukla, J P Jaiswal,Ishwar Singh Solanki, G P Singh and A Mahendru-Singh*(2021).Identification of Novel Genomic Regions for Biofortification Traits Using an SNP Marker-Enriched Linkage Map in Wheat (Triticumaestivum).Front.Nutr., https:/ /doi.org/10.3389/fnut.2021.669444
- Kumar, T P J, A Rai, S K. Singh, R R Kumar, A K Ahlawat, S Saini, R B Shukla, N Bedi and A Mahendru-Singh* (2021). Development of near isogenic lines for grain softness through marker assisted backcross breeding in wheat. Journal of Plant Biochem. https://doi.org/10.1007/s13562-021-00712-x
- Soumya P R, D Singh, S Sharma, A Mahendru-Singh and R Pandey (2021). Evaluation of Diverse Wheat (Triticum aestivum) and Triticale (× Triticosecale) Genotypes for Low Phosphorus Stress Tolerance in Soil and Hydroponic Conditions. J Soil Sci Plant Nutr. 21, 1236–1251. https://doi.org/10.1007/s42729-021-00436-w
- Singh N, A S Virdi, M Katyal, A Kaur, D Kaur, A K Ahlawat, Anju Mahendru-Singh and R K Sharma (2021). Evaluation of heat stress through delayed sowing on physicochemical and functional characteristics of grains, whole meals and flours of India wheat. Food Chemistry: 344, 1218725; https://doi.org/10.1016/j.foodchem.2020.128725
- Kaur N, B Singh , A Kaur , M P Yadav, N Singh, A K Ahlawat, Anju Mahendru-Singh (2021).Effect of growing conditions on proximate, mineral, amino acid, phenolic composition and antioxidant properties of wheatgrass from different wheat (Triticum aestivum) varieties. 2021. Food Chemistry, 341(1): 121; https://doi.org/10.1016/j.foodchem.2020.128201
- Rai, A, Arvind K. Ahlawat, R. B. Shukla, N Jain, Rajeev Ranjan Kumar and A Mahendru-Singh*(2021). Quality evaluation of near‑isogenic line of the wheat variety HD2733 carrying the Lr24/Sr24 genomic region. Biotech-3: 11, 130
- Sharma, S, M Katyal, N Singh, A Mahendru-Singh and A K Ahlawat. (2021). Comparison of the effect of using hard and soft wheat on molecular weight glutenin subunits profile and quality of produced cookie. J Food Sci and Technology: http://doi.org/10.1007/s13197-021-05272-5.
- Radhamani J, H Pushpa, SS Gomshe, SB Choudhary, V Kumar, R Bisen and M Sujatha (2021) Early maturing niger germplasm accessions. Ind. Jour. Plant Gene. Resour. 34: 495–98. https://doi.org/10.5958/0976-1926.2021.00045.0. (NAAS Rating-5.17)
- Bansal S, K Sharma, V Gautam, AA Lone, EV Malhotra, S Kumar &R Singh (2021) A comprehensive review of Bunium persicum: a valuable medicinal spice. Food Reviews International, 39(2): 1184-1202 DOI: 10.1080/87559129.2021.1929305 (NAAS Rating-11.8)
- Ghosh D, CR Chethan, S Chander, B Kumar, RP Dubey, HS Bisen, SK Parey & PK Singh (2021) Conservational Tillage and Weed Management Practices Enhance Farmers Income and System Productivity of Rice–Wheat Cropping System in Central India. Agricultural Research, 1-9. (NAAS Rating-7.4)
- Gowthami R, N Sharma, KK Gangopadhyay, S Rajkumar, P Pathania & A Agrawal (2021) Cryopreservation of pollen of Abelmoschus moschatus medik. subsp. moschatus as an aid to overcome asynchronous flowering for wide hybridization with cultivated okra [A. esculentus (L.) Moench]. CryoLetters, 42(4): 233 – 244. (NAAS Rating-7)
- Gowthami R, C Vanniarajan, J Souframanien, K Veni & VG Renganathan (2021) Efficiency of electron beam over gamma rays to induce desirable grain-type mutation in rice (Oryza sativa L.). International Journal of Radiation Biology, 97(5): 727-736. (NAAS Rating-8.6)
- Malhotra EV, M Kamalapriya, S Bansal, DPS Meena, A Agrawal (2021). Improved protocol for micropropagation of genetically uniform plants of commercially important cardamom (Elettaria cardamomum Maton). In Vitro Cellular & Developmental Biology – Plant, 57: 409–417 (NAAS Rating-8.6)
- Malhotra EV, R Jain, S Bansal, SC Mali, N Sharma, A Agrawal (2021). Development of a new set of genic SSR markers in the genus Gentiana: in silico mining, characterization and validation, 3 Biotech, 11: 430 (NAAS Rating-8.8)
- Malhotra EV, S Bansal, P Pathania, SC Mali, A Agrawal (2021) Regeneration of plantlets through protocorm-like bodies: An alternative method for vanilla micro propagation. Indian Journal of Biotechnology, 20: 388-395 (NAAS Rating-6)
- Sharma N, R Gowthami, S Vimala Devi, EV Malhotra, R Pandey & A Agrawal (2021). Cryopreservation of shoot tips of Gentiana kurroo Royle – a critically endangered medicinal plant of India. Plant Cell, Tissue and Organ Culture, 144:67-72 (NAAS Rating-9)
2020
- Akhtar J, B Singh, R Kiran, P Kumar, M Shekhar, Sadhana, S Pandey, S Lenka and SC Dubey (2020). Studies on infection indexing and distribution profiling of seed-borne fungi of sorghum germplasm in India for safe and healthy long-term conservation. Indian J. Plant Genet. Resour. 33(3): 334-346 (NAAS Rating-5.54)
- Gupta Veena (2020) Conserving trait specific germplasm of medicinal plants–catering to needs of pharmaceutical industries. Journal of Plant Development Sciences . 12(7): 443-448. 2020. (NAAS Rating-4.35)
- Rathan, N, A Mahendru-Singh*, V Govindan and M I Ibba(2020). Impact of High and Low-Molecular-Weight Glutenins on the Processing Quality of a Set of Biofortified Common Wheat (Triticum aestivum L.) Lines. Front. Sustain. Food Syst., https:// doi. org/ 10.33 89/fsufs.2020.583367.
- Azizi, A F, S Sethi, A Joshi, A Mahendru-Singh, P Raigond, M K Singh and R K Yadav (2020). Biochemical and functional attributes of raw and boiled potato flesh and peel powders for suitability in food applications. J Food Sci Technol,57, 3955–3965.https://doi.org /10. 1007/s1 3197-020-04424-3.
- Jacob SR, Mishra A, K Manju, Bhatt K, Gupta V and K Singh. (2020). A Quick Viability Test Protocol for Hemp (Cannabis Sativa L.) Seeds. Journal of Natural Fibers. 1-6. 10.1080/15440478.2020.1764451. (NAAS Rating-9.5)
- Malav Pavan Kumar, Anjula Pandey, Veena Gupta, Ashok Kumar, KC Bhatt, Archana Raina, Jameel Akhtar, Ambika Gaikwad, Ombir Singh Ahlawat and Rajkumar Dhakad. 2020. Diversity in Holy Basil (Ocimum tenuiflorum L.) Germplasm from India. Indian Journal of Agricultural Sciences. Vol. 90 (10): 1937-45. (NAAS Rating- 6.25). (NAAS Rating-6.4)
- Malav Pavan Kumar, R Bhardwaj, Anjula Pandey and Veena Gupta. 2020. African Bitter Leaf [Vernonia amygdalina Delile]: Study on Seasonal Variations in Total Phenols and Seed Germination in India. Indian J. Plant Genet. Resour. 33(2): 187–191 (2020) DOI 10.5958/0976-1926.2020.00027.3. (NAAS Rating-5.54)
- C.,Gayacharan, Archak Sunil , Gupta Kavita, Gupta Veena, Tyagi Vandana and Kuldeep Singh (2020). Mungbean Genetic Resources and Utilization. 10.1007/978-3-030-20008-4_2.
- Nanjundan J, C Manjunatha, J Radhamani, AK Thakur, R Yadav, A Kumar, ML Meena, RK Tyagi, DK Yadava and D Singh (2020) Identification of new source of resistance to powdery mildew of indian mustard and studying its inheritance. Plant Pathol. J. 36: 111–20. https://doi.org/10.5423/PPJ.OA.07.2019.0205. (NAAS Rating-8.3)
- Vikas VK, S Kumar, S Archak, RK Tyagi, J Kumar, S Jacob, M Sivasamy, P Jayaprakash, MS Saharan, AK Basandrai, D Basandrai, K Srinivasan, J Radhamani, R Parimalan, S Tyagi, J Kumari, AK Singh, J Peter, R Nisha, M Yadav, J Kumari, HK Dhillon, D Chauhan, S Sharma, S Chaurasia, RK Sharma, M Dutta, GP Singh and KC Bansal (2020) Screening of 19,460 genotypes of wheat species for resistance to powdery mildew and identification of potential candidates using focused identification of germplasm strategy (FIGS). Crop Sci. 60: 2857–66. https://doi.org/10.1002/csc2.20196. (NAAS Rating-8.3)
- Gupta V, PG Gore, A Kak and J Aravind (2019) Breaking seed dormancy in Cleome viscosa L. for improving seed germination. Med. Plnts. Int. Jrnl. Phyt. Rela. Ind. 11: 203. https://doi.org/10.5958/0975-6892.2019.00027.3. (NAAS Rating-5.25)
- Singh N, PG Gore and J Aravind (2020) Breaking seed coat impermeability to aid conservation and utilization of wild Vigna species. Genet. Resour. Crop Evol. 67: 523–29. https://doi.org/10.1007/s10722-019-00872-9. (NAAS Rating-8)
- Dudhe MY, M Sujatha, HP Meena, MK Ghodke, YG Shadakshari, J Radhamani, K Alivelu, RK Tyagi, ARG Ranganatha and KS Varaprasad (2020) Sunflower germplasm catalogue: Necessity and benefit to sunflower researchers. J. Oilseeds Res. 37:. India Society of Oilseeds Research: 75. (NAAS Rating-4.78)
- Chaudhury R, M Shankar, Rampal, M Awasthi, B Thongam, SK Malik & H Pritchard (2020) Seed Cryopreservation of Orchid Coelogyne nitida (Wall. ex Don) Lindl. Using Air Desiccation and Vitrification. Indian Journal of Plant Genetic Resources, 33(2): 146-153 (NAAS Rating-5.17)
- Chethan CR, PK Singh, RP Dubey, S Chander, D Ghosh, VK Choudhary & RK Fagodiya (2020) Crop residue management to reduce GHG emissions and weed infestation in Central India through mechanized farm operations. Carbon Management, 11(6):565-76 (NAAS Rating-9.1)
- Gupta S & Pradheep K (2020) Diversity, conservation and use of underutilized and minor fruits in India: an overview. Acta Horticulturae, 1297: 51-60.
- Gupta S & Tewari P (2020) Cryopreservation of Fragaria × ananassa using different techniques. Acta Horticulturae, 1298: 161-166.
- Gupta S, DB Parakh & SK Verma (2020) Ex situ conservation of strawberry (Fragaria spp.) germplasm in ICAR-NBPGR. Acta Horticulturae, 1297: 89-98.
- MR Rohini, M Sankaran, S Rajkumar, K Prakash, A Gaikwad, SK Malik, R Choudhary (2020) Morphological characterization and analysis of genetic diversity and population structure in Citrus× jambhiri Lush. using SSR markers. Genetic Resources and Crop Evolution 67(5), 1259-1275 (NAAS Rating-8)
- Sharma N, R Gowthami, R Pandey & A Agrawal (2020) Influence of explant types, non-embryogenic synseed and reduced oxygen environment on in vitro conservation of Bacopa monnieri (L.) Wettst. In Vitro Cellular Developmental Biology- Plant, 56: 851–856. (NAAS Rating-8.6)
- Singh K, SK Malik, S Gupta & R Chaudhury (2020) Unlocking genebanks to ensure food and nutrient security and environmental stability. Acta Horticulturae, 1297: 1-8.
- Malik, S K, R Choudhary, S Kaur, R Chaudhury & HW Pritchard (2020) Storage behavior and cryopreservation of Citrus cavaleriei, an endangered, cold-resistant species of northeast India with exceptionally large seeds. CryoLetters, 41(5): 281-290. (NAAS Rating-7)
- Srivastava V, DK Nerwal, A Kandan, J Akhtar, N Sharma, R Kiran, S Bansal & A Agrawal (2020) Management of microbial contaminants in the In Vitro Gene Bank: a case study of taro [Colocasia esculenta (L.) Schott]. In Vitro Cellular & Developmental Biology – Plant. doi.org/10.1007/s11627-020-10125-5 (NAAS Rating-8.6)
2019
- Gupta Veena, Padmavati G Gore and Anjali Kak (2019) Collection, conservation and utilization of indigenous ornamental crops. Indian horticulture, 64 (4): 76-81.
- Gore Padmavati G., Bhardwaj Rakesh, Gupta Veena. 2019. Effect of different drying methods on chlorophyll, carotenoids and organoleptic characteristics of curry leaves. Medicinal Plants – International Journal of Phyto medicines and Related Industries. Vol 11(2) : 200-202 (NAAS Rating-5.25)
- Singh M, G Randhawa, RK Bhoge, S Singh, A Kak and O Sangwan (2019) Monitoring adventitious presence of transgenes in cotton collections from genebank and experimental plots: ensuring gm-free conservation and cultivation of genetic resources. Agric. Res. doi: 10.1007/s40003-019-00449-z. (NAAS Rating-7.4)
- Gupta V, P G Gore and A Kak (2019) Collection, conservation and utilization of indigenous ornamental crops. Indian Horticulture 64(4): 76-81.
- Katyal, M, K N Singh,A S Virdi, A Kaur, AK Ahlawat, A Mahendru-Singh and R Bajaj. (2019). Comparative analysis of native and defatted flour from hard, extraordinarily soft, and medium‐hard wheat varieties for protein solvation, pasting, mixing, and dough rheological behaviour. Journal of Food Science, 85(1), https://doi.org/10.1111/1750-3841.14944.
- Rai, A, A Mahendru-Singh*, A, Ahlawat, A, K, Kumar, K, Raghunandan, S. Saini, D Ganjewala and R B Shukla. (2019). Quality evaluation of near isogenic lines of the wheat variety carrying Sr26, Lr19 and Yr10 genes. Journal of Cereal Science. 88: 110-119.
- Rai, A, Mahendru-Singh*, K, Raghunanadan, TPJ, Kumar, P Sharma, AK Ahlawat, SK Singh, D Ganjewala, RB, Shukla and M Shivasamy. (2019). Marker assisted transfer of PinaD1a gene to develop soft grain wheat cultivars. Biotech-3, 9:183-189.
- Rai, A, A Mahendru-Singh*, D Ganjewala, R R Kumar, A K Ahlawat, S K Singh, P Sharma and N Jain (2019). Rheological evaluations and molecular marker analysis of cultivated bread wheat varieties of J Food Sci. Techno,. 56: 1696-1707https://doi.org/10.1007/s13197-019-03593-0.
- Krishnappa, G., Ahlawat, A.K., Shukla, R B, Singh, S K, Anju M Singh*, and Singh G P. (2019). Multi-environment Analysis of Grain Quality Traits in Recombinant Inbred Lines of a Biparental Cross in Bread Wheat (Triticum aestivum). Cereal Res. Commun. 47:334-344.
- Krishnappa G, K Srinivasan, A Mahendru-Singh*, A Mishra, A K Ahlawat, G P Singh and S Rachel-Jacob (2019). Exploratory studies on components of variability for seed longevity and quality traits in bread (Triticum aestivum) and durum (Triticum durum) wheat, Ind JAgri Sci, 89(3): 515–21.
- Ranjan, R, Yadav, R, Pandey, R, Jain, N, Bainsla, N, Gaikwad, K & Anju M Singh (2019).Variation in wheat (Triticum aestivum) advance lines and released cultivars for traits associated with nitrogen use efficiency under N limiting environment. Ind J Agri Sci, 89: 99-104
- Nisa V, W Nisa, ZA Dar, MA Wani, V Gupta, A Kak and SR Jacob (2019) Impact of climate change on maize productivity The Pharma Innovation Journal 8(2): 83-94
- Padmavati G. Gore, A.Kak and J.Aravind (2019) Breaking seed dormancy in Cleome viscosa L. for improving seed germination. Medicinal Plants11:117-119. (NAAS Rating-5.25)
- Babu PK, J Radhamani, S Rajkumar and R Tyagi (2019) Note on diversity in legumes and oilseeds and their wild relatives in eastern ghats of india: Utilization and conservation concerns. Ind. Jour. Plant Gene. Resour. 32: 43. https://doi.org/10.5958/0976-1926.2019.00006.8. (NAAS Rating-5.17)
- Bansal S, M Mangal, S Tushir, HS Oberoi, RK Gupta (2019) A rapid and reliable method for the specific detection of aflatoxigenic fungi in groundnut and rice samples. Journal of Food Processing & Preservation, 43(10)e14127: 1-6 (NAAS Rating-8.5)
- Bansal S, S Thakur, M Mangal, AK Mangal & RK Gupta (2019) Identification of suitable locus for specific detection of biological adulterants of saffron. Food Analytical Methods, 12: 2509–2517 (NAAS Rating-8.9)
Projects
Institute Funded Projects
Project: Ex-situ Conservation of Agricultural and Horticultural Genetic Resources in the National Genebank
Project Leader: Dr Anju Mahendru Singh
Sub-Project 1: Ex-situ Conservation of cereals, legumes, oilseeds and potential crops and their wild relatives in the Seed Genebank (sub-project PI: Dr Anju Mahendru Singh)
Sub-Project 2: Ex-situ Conservation of vegetables, millets, fiber, forages, medicinal and aromatic, ornamentals, narcotics, spices, fruits, nuts and their wild relatives in the seed Genebank (Sub-project PI: Dr Anjali Kak Koul)
Sub-Project 3: Ex-situ Conservation of vegetatively propagated crops in the in vitro Genebank (Sub-project PI: Dr Sandhya Gupta)
Sub-Project 4: Ex-situ Conservation of Agri-Horticultural crops and their wild relatives in the Cryogenebank (Sub-project PI: Dr S K Malik)
Sub-Project 5: Maintenance and Information Management of National Germplasm Conservation Network (Sub-Project PI: Dr Hanuman Lal)
Externally Funded Projects
Funding Agency & Duration | Project cost | Project title | PI/Co PI/ Associates | Research Staff |
PPV&FRA Continuous since 2007 | 186.89 Lakhs | Implementation of legislation on Protection of Plant Varieties and Farmers’ Rights | Sherry R Jacob | SRF-1 |
SERB, DST, GoI (2023-2026) | 42.82192 Lakhs | Cryobiotechnological approaches …… for Base Germplasm Collection of Indian Piper Species | Era Vaidya Malhotra, Scientist (SS) | Nil |
DBT, GoI (2020-2025) | 94.1516 Lakhs | Leveraging genetic resources for accelerated genetic improvement of Linseed using comprehensive genomics and phenotyping approaches” under mission programme of Minor Oilseeds of Indian Origin” (Sub-project 6; Component-1) | Vartika Srivastava, Scientist (SS), (Co- PI) | Nil |
DBT, GoI (2021-2024)
| 74.3272 Lakhs | Collection, Conservation and Morpho-Phenological Characterization of Citrus Germplasm of North East
| S. K. Malik, PS, (PI), Sangita Bansal, PS, (Co-PI)
| JRF – 1 Lab Assistant – 1 Field Assistant – 1 |
Staff
Scientist

Head of Division
Division of Germplasm Conservation
Phone: 011-25802784
Email: anju.singh(AT)icar.org.in; nbpgr.conservation(AT)gmail.com

Principal Scientist
Division of Germplasm Conservation
Phone: 011-25802721
Email: sandhya.gupta(AT)icar.org.in, sandhya_gupta87(AT)yahoo.com

Principal Scientist
Division of Germplasm Conservation
Phone: 011-25802773
Email: Anjali.Koul(AT)icar.org.in

Principal Scientist
Division of Germplasm Conservation
Phone: 011-25802715
Email: nidhiverma.icar@gmail.com; nidhipgr@gmail.com

Principal Scientist
Division of Germplasm Conservation
Phone: 011-25802772
Email:Chithra.Pandey(AT)icar.org.in, chitra_nbpgr(AT)rediffmail.com

Principal Scientist
Division of Germplasm Conservation
Phone: 011-25802703
Email: Sushil.Pandey(AT)icar.org.in

Principal Scientist
Division of Germplasm Conservation
Phone: 011-25802788
Email:Sherry.Jacob(AT)icar.org.in, sherryrachel(AT)yahoo.co.in

Senior Scientist
Division of Germplasm Conservation
Phone: 011-25802713
Email: subhash.chander3(AT)icar.org.in, singhariya43(AT)gmail.com

Senior Scientist
Division of Germplasm Conservation
Phone: 011-25802808
Email: Era.Vaidya(AT)icar.org.in, vaidya.era(AT)gmail.com

Senior Scientist
Division of Germplasm Conservation
Phone: 011-25802778
Email:suman.roy(AT)icar.org.in, Rsuman2714(AT)gmail.com

Senior Scientist
Division of Germplasm Conservation
Phone: 011-25802775
Email: Vartika.Srivastava(AT)icar.org.in, vartika0906(AT)gmail.com

Senior Scientist
Division of Germplasm Conservation
Phone: 011-25802776
Email: Padmavati.Gore(AT)icar.org.in, padma.pgr(AT)gmail.com

Scientist
Division of Germplasm Conservation
Phone: 011-25802765
Email:j.aravind(AT)icar.org.in

Scientist
Dr. Deepika DD
Division of Germplasm Conservation
Phone:
Email: deepika.dd96-icar@nic.in
Technical

Assistant Chief Technical Officer
Division of Germplasm Conservation
Phone: 011-25802706
Email:Rajvir.Singh1(AT)icar.org.in, rajvir71(AT)rediffmail.com

Sr. Technical Officer
Division of Germplasm Conservation
Phone:
Email: Satya.Prakash1(AT)icar.org.in

Technical Officer
Division of Germplasm Conservation
Phone:
Email: Lal.Singh2(AT)icar.org.in

Senior Technical Assistant
Division of Germplasm Conservation
Phone: 011-25802766
Email:Anjali(AT)icar.org.in, anjalisingh14.10.92(AT)gmail.com

Senior Technical Assistant
Division of Germplasm Conservation
Phone: 011-25802234
email: suresh.mali(AT)icar.org.in, mali.123pbg(AT)gmail.com

Senior Technical Assistant
Division of Germplasm Conservation
Phone:011-25802766
Email: neha.sharma1(AT)icar.org.in, bioinformatics.neha(AT)gmail.com
Supporting

Skilled Supporting Staff
Mrs. Geeta Devi
Division of Germplasm Conservation
Phone:
Email: Geeta.Devi1(AT)icar.org.in

Skilled Supporting Staff
Mr. Nand Kishore
Division of Germplasm Conservation
Phone:
Email: Nand.Kishore2(AT)icar.org.in


